3134
Feasibility of Integrating 7 Diffusion Models within a Single Acquisition and Their Performance Comparison in Glioma Grading1Shanghai University of Medicine & Health Sciences, Shanghai, China, 2MR Scientific Marketing, Siemens Healthineers, Shanghai, China, 3Department of MRI, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 4Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science,East China Normal University, Shanghai, China
Synopsis
There are many advanced diffusion models available for neural and body researches, while their acquisitions are different and normally difficult to be joint applied and compared in one study. In this study, we tried to calculate 7 diffusion models, including 4 neural and 3 cancer related models, using data from one acquisition, and applied it for glioma. Two diffusion acquisition strategy were evaluated, and the performance of these models in glioma grading was also compared. The result showed that both acquisition strategies could generate high quality quantitative parameters for all models, which showed significant differences between high- and low-grade tumor.
Introduction
Advanced diffusion models are widely used in brain white/gray matter cancer studies. The popular neural diffusion models include diffusion tensor imaging (DTI), diffusion kurtosis imaging (DKI), neurite orientation dispersion and density imaging (NODDI) and mean apparent propagator (MAP) models. In our previous work, we applied multiple neural diffusion models in diagnosis of epilepsy, prediction of glioma grading and gene status (1, 2), and found many quantitative parameters from these models showed significant performance, especially NODDI and MAP models. To speedup acquisition, all these models could be calculated using a single DSI acquisition.Besides neural diseases, another main application of diffusion is cancer diagnosis and prognosis, and a number of cancer related models were available, such as intra-voxel-incoherent-motion (IVIM) model, stretch exponential model (SEM), fractional-order calculus (FROC), Continuous-Time-Random-Walk (CTRW). They provide perfusion and tumor heterogeneity information, and show great potential in cancer. The feasibility of joint application of these models and the comparison between cancer and neural related models are not clear. In this study, we tried to calculate 7 diffusion models, including 4 neural models and 3 cancer related models, by data from one acquisition, and applied it for glioma. Two diffusion acquisition strategy were evaluated, and the performance of these models in glioma grading was also compared.
Methods
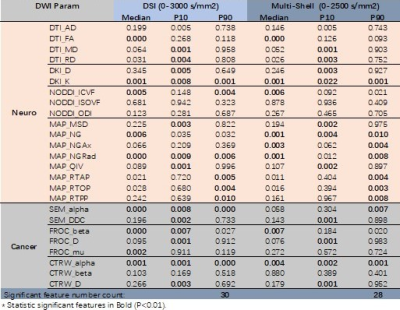
Totally 49 glioma patients underwent MRI scan using a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). There are 1, 22, 5 and 21 patients at glioma grade 1, 2, 3, 4, respectively. Grade 1 and 2 was considered as low-grade, and grade 3 and 4 as high-grade. The diffusion data were acquired using two strategies: 1) a DSI scheme with fully Cartesian q-space grid design and q-space factor 3. Totally two b=0 data and 98 DWI data with 11 nonzero b-value (0~3000 s/mm2) were acquired within one scan. The other imaging parameters were as follows: TR/TE = 7300/93 ms, FOV = 300 × 300 mm2, matrix = 128 × 128, slice thickness = 2.5 mm, in-plane acceleration factor = 2. 2) a multi-shell scheme with 5 non-zero b-values (500, 1000,1500,2000,2500 s/mm2) and 30 gradient directions at each b value.The parameters of neural diffusion models, namely DTI, DKI, NODDI and MAP-MRI, were calculated using an in-house developed software called NeuDiLab, which is based on an open-resource tool DIPY (Diffusion Imaging In Python, http://nipy.org/dipy). The parameters of cancer related diffusion models, namely SEM, FROC and CTRW models, were calculated using another in-house developed software called BoDiLab, which is based on Python 3.7. All the DWI data were used in model estimation. Region-of-interest (ROI) was draw on T2 weighted image covering the whole tumor and transferred to DWI parameters by rigid registration using Elastix. A few histogram features were extracted, namely median and 10% and 90% percentiles values, which showed high performance in our previous study [radiology 2021]. Two sample t-test was performed with P < 0.01 as statistical significance.
Results
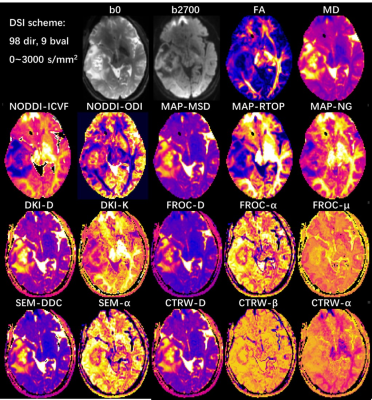
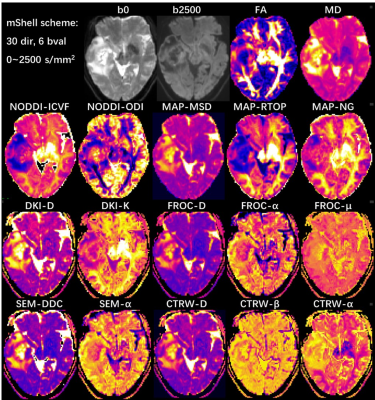
All neural and cancer related models were successfully estimated. Figure 1 and 2 shows parameter maps of 7 diffusion models from a glioma patient based on DSI and multi-shell acquisition schemes respectively, with similar image quality. In quantitative analysis, DSI and multi-shell schemes generated also similar performance in glioma grading, with 30 and 28 features reaching statistical significance respectively. Among models, most of the parameters showed statistical significance in tumor grading, especially for DKI_K, MAP_NGRad, CTRW_alpha that most of the features showed high performance.Discussion
In this study, we found that simultaneously applying 7 neural or cancer related diffusion models is feasible based on one single acquisition, and could be successfully applied in glioma grading. The result showed both two acquisition schemes can successfully generate diffusion parameters of all 7 models, and the parameters showed similar image quality. We could classify these parameters into several group according to their physical meaning:1) the non-gaussian related parameters DKI_K, MAP_NG; 2) intracellular diffusion parameters NODDI_ICVF, MAP_RTOP; 3) tumor heterogeneity related parameters SEM_alpha, FROC_alpha, CTRW_alpha; 4) ADC like parameters DTI_MD, DKI_D, MAP_MSD, SEM_DDC, FROC_D, CTRW_D. All these parameters reflected different aspect of tissue and generate multi-dimensional information, which could be further combined to build predicting model for high performance.Meanwhile, the idea to joint apply multiple diffusion models could be extend to more diseases, including neural and also body cancer diseases. The neural application will be straightforward. In body application, either the performance of neural models or joint usage of multiple cancer related models could be investigated, which will depend on different acquisition scheme, with or without high number of diffusion gradient directions. While in either case, a single and comprehensive acquisition allowing joint application of multiple models will significantly reduce scan time and make it more feasible in clinical practice. Our result indicated that it is feasible under two commonly used acquisition schemes.
To conclude, this study showed that simultaneous applying 7 neural and cancer related diffusion models was feasible under a single diffusion acquisition, and the results showed that most of the diffusion parameters showed statistical significance in high- and low-grade glioma diagnosis. In addition, multiple acquisition scheme could be used to achieve similar results, which make this idea easy to apply in future study.
Acknowledgements
No acknowledgement found.References
1. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC: NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 2012; 61:1000–1016.
2. Fick RHJ, Wassermann D, Caruyer E, Deriche R: MAPL: Tissue microstructure estimation using Laplacian-regularized MAP-MRI and its application to HCP data. Neuroimage 2016; 134:365–385.
3. Ma K, Zhang X, Zhang H, et al.: Mean apparent propagator-MRI: A new diffusion model which improves temporal lobe epilepsy lateralization. Eur J Radiol 2020; 126.
4. Le H, Zeng W, Zhang H, et al.: Mean Apparent Propagator MRI Is Better Than Conventional Diffusion Tensor Imaging for the Evaluation of Parkinson’s Disease: A Prospective Pilot Study. Front Aging Neurosci 2020; 12.
Figures