3127
Infarct core estimation with ADC: A retrospective study1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Department of Radiology, Shanghai Fourth People's Hospital, School of Medicine, Tongji University, Shanghai, China, 31Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University 2Human Phenome Institute, Fudan University, Shanghai, China
Synopsis
According to a large amount of emergency and follow-up data, we propose an optimized threshold of the apparent diffusion coefficient to identify the ischemic core with fair accuracy, which is crucial for the decision of the treatment scheme and operation mode.
Introduction
Stroke is a worldwide problem and the leading cause of disability today.1,2,3. Increasing morbidity and mortality rates of cerebral infarction have placed a high socioeconomic burden on patients and family members. During routine emergency diagnosis, physicians need to make a precise estimation of the infarct core location and area to decide the treatment scheme and operation mode.Diffusion-Weighted Imaging (DWI) is an imaging modality widely used for the diagnosis and management of acute cerebral infarction. In ischemic tissue, cytotoxic edema, where water molecules in tissue diffuse, leads to a reduction in the apparent diffusion coefficient (ADC). 4,5 The region of infarct core, defined as a low-density area on ADC, is an important indicator for the differentiation of salvage methods.
The previous study suggested an optimal threshold for the identification of ischemic core with an apparent diffusion coefficient ≤620×10 -6 mm2/s.6 In practical application, this criterion is not accurate. The area screened with this criterion was larger than the true core infarct area after healing. This results in patients not receiving the best treatment outcome. Therefore, the purpose of this study was to propose a core infarct zone threshold applicable for the Asian population to guide the selection and implementation of treatment protocols.
Methods
A large amount of real emergency and follow-up data is the basis of this study. A total of 349 patients with acute cerebral ischemic stroke between March 2013 and January 2000 were retrospectively recruited. Patients underwent brain MRI within 24 hours of ischemic stroke onset and were imaged more than twice with at least a 3-month time interval. MRI examinations were obtained with a 1.5-T MR unit (Avanto, Siemens).Accurate and automated 3D geometrical registration of images is vital for the understanding of the changes in areas of interest. The final true infarct core area was standardized by the region in the T2 imaging and aligned with the T1, Flair to improve diagnostic accuracy. ADC within 24 hours onset and T1, T2, T2 FLAIR scans after 3 months were coregistered with FSL Tools.
Two independent specialists annotated the ischemic core on follow-up images with ITK-SNAP software. Based on these annotations, masked images have been created, which are used to check the value of ADC of ROI. Afterward, A voxel-based statistical analysis scheme was used.
Results
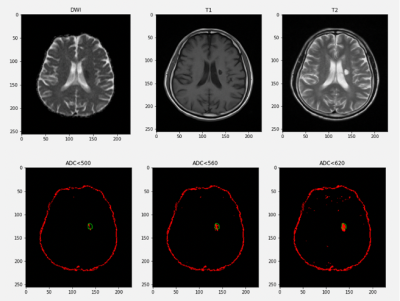
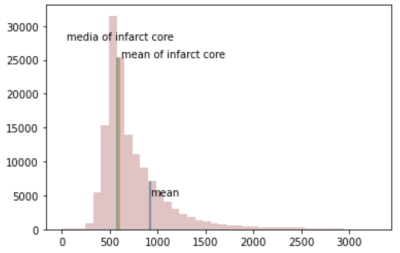
The area of the final infarct core should be larger than the area we estimated from the ADC image. As shown in figure 1, in this case, when the estimation threshold was set to 620 ×10-6mm2/s, the estimation space exceed the edge of the true core infarct, which means that this threshold is not accurate.With the statistical analysis method, the distribution of annotated core infarct intensity is shown in figure 2. As the ischemic core expand, parts of the areas, which belong to the ischemic penumbra 7,8 were included in the mask region so that hyperintensity appeared in the figure. When volume with intensity larger than average volume intensity was excluded, the average and mediate of ADC signal of all patients are 604 ×10-6mm2/s and 583 ××10-6mm2/s. After a lot of practice and application of proven, the final threshold of infarct core on ADC was obtained as a mean of 581 ×10-6mm2/s.
Discussion and conclusion
By using a statistical approach, we can see that the previous criteria were inaccurate for the patients in our study. Therefore propose an optimized ADC threshold of 581 ×10-6mm2/s to identify the ischemic core with fair accuracy. Subsequent work will focus more on identifying true core infarct areas with DWI image data in the emergency setting to improve treatment outcomes.Acknowledgements
No acknowledgement found.References
1. Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders, and prognosis. Current Cardiol Rev. 2010;6:150–61.
2. Weir NU. An update on cardioembolic stroke. Postgrad Med J. 2008;84:133–42.
3. Ferro JM. Brain embolism. Answers to practical questions. J Neurol. 2003;250:139–47.
4. Straka M, Albers GW, Bammer R. Real-time diffusion-perfusion mismatch analysis in acute stroke. J Magn Reson Imaging 2010; 32:1024– 37.
5. Bratane BT, Bastan B, Fisher M, Bouley J, Henninger N. Ischemic lesion volume determination on diffusion-weighted images vs. apparent diffusion coefficient maps. Brain Res 2009; 1279:182– 8.
6. Archana Purushotham, Bruce C. V. Campbel, Matus Straka4, Michael Mlynash1, Jean-Marc Olivot1, Roland Bammer4, Stephanie M. Kemp1, Gregory W. Albers1, and Maarten G. Lansberg. Apparent diffusion coefficient threshold for delineation of ischemic core. International Journal of Stroke 2015,10,3:348-353.
7. Lo EH: A new penumbra: transitioning from injury into repair after stroke. Nat Med 14:497–500, 2008
8. PaciaroniM, CasoV, Agnelli G: The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol 61:321– 330, 2009