3126
Stage-dependent differential influence of metabolic and structural networks on memory across Alzheimer’s disease continuum1Centre for Sleep and Cognition and Centre for Translational MR Research, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore, Singapore, Singapore, 2Department of Neurology, National Neuroscience Institute, Singapore, Singapore, Singapore, 3Duke-NUS Medical School, Singapore, Singapore, Singapore, 4Lee Kong Chian School of Medicine, Nanyang Technological University Singapore, Singapore, Singapore, Singapore, Singapore, 5Translational Neuroimaging Laboratory, The McGill University Research Centre for Studies in Aging, Montreal, Canada, Montreal, QC, Canada, 6Alzheimer’s Disease Research Unit, The McGill University Research Centre for Studies in Aging, McGill University, Montreal, Canada, Montreal, QC, Canada, 7Department of Electrical and Computer Engineering, National University of Singapore, Singapore, Singapore, Singapore, Singapore, 8Integrative Sciences and Engineering Programme (ISEP), National University of Singapore, Singapore, Singapore, Singapore, Singapore
Synopsis
While emerging evidence suggests the association between network neurodegeneration and memory varies with pathology in Alzheimer’s disease (AD), the trajectory of this relationship remains elusive. We stratified 708 participants into non-amyloid/non-tau, tau-only, and AD pathology groups and examined the associations between individual-level structural and metabolic network integrity and memory across cognitive stages (cognitively normal, mild cognitive impairment, and probable AD) in each pathology group. The associations of hippocampal and default mode networks with memory exhibited differential pathology-dependent trajectories across cognitive stages. Our findings pave the way for early interventions and stage-dependent remedies to modify disease trajectory and improve clinical outcomes.
INTRODUCTION
Alzheimer’s disease (AD) is a neurodegenerative disease that is characterized by neuropathological changes of amyloid-beta (Aβ) plaques (A), intraneuronal tau neurofibrillary tangles accumulation (T), and neurodegeneration (N) which represents neuronal injuries in the forms of cerebral grey matter (GM) atrophy and hypometabolism in the brain [1, 2]. While large-scale neuronal network breakdown underlies memory impairment [3], the relationships between changes in individual-level network-based neurodegeneration across different AD pathology groups and cognitive stages, and their influence on memory impairment, remain unclear. Here, we sought to determine the differential associations of brain structural and metabolic covariance network integrity with memory performance among cognitively normal (CN), mild cognitive impairment (MCI), and probable AD individuals stratified by their A and T biomarker status, without assuming a constant relationship.METHODS
708 participants (195 CN, 374 MCI, and 139 probable AD) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) were stratified based on CSF Aβ1-42 (A) and p-tau181p (T) into A-T- (non-amyloid/non-tau), A-T+ (tau only), and A+T-/A+T+ (AD pathology) groups. Given our focus on memory performance and AD pathology, we selected a set of seeds from the three higher-order cognitive networks (including the default mode network (DMN), salience network, executive control network (ECN)) as well as the hippocampus (HIP)-based memory network based on the group comparisons of the grey matter volume (GMV) probability and glucose metabolic spatial maps between CN and probable AD individuals. Using seed-based partial least square analyses [4], we derived the individual-level structural and metabolic covariance network score of these networks, which reflected how strongly each brain network pattern was manifested in the individual’s metabolic and structural brain networks (Fig. 1). Rather than assuming constant contributions of brain network integrity to memory impairment in different pathology groups across the three cognitive stages (CN, MCI, and probable AD), we characterized the nonlinear associations of brain metabolism and GMV covariance network scores with memory across the stages in different Aβ/tau pathology groups using the sparse varying coefficient (SVC) model [5], which also allows the selection of significant predictors with the LASSO sparse penalty while eliminating the contribution of the less important predictors.RESULTS
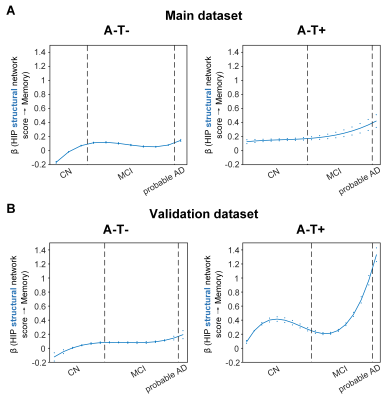
Overall, individual-level brain structural and metabolic network scores were lower in individuals with worse cognition and AD pathology. The SVC modelling identified the HIP structural network score as a key predictor of memory impairment in all three pathology groups, and the angular gyrus (ANG) metabolic network score to be associated with memory impairment only in the AD pathology (A+T-/A+T+) group. The associations of structural and metabolic networks in the hippocampal and ANG-seeded default mode regions with memory performance exhibited pathology-dependent differential trajectories across cognitive stages (Fig. 2 and 3). Specifically, in the AD pathology group, the association between hippocampal structural network and memory impairment was strongest in early CN and gradually decreased from late CN to dementia, suggesting an early influence of hippocampal structural network deterioration on memory impairment in asymptomatic amyloid-positive individuals. In contrast, such hippocampus-memory association was weaker overall in non-amyloid/non-tau and tau only groups, which was lowest in early CN and gradually increased from MCI to dementia. We observed the same pathology-dependent differentiation for metabolic covariance networks. In AD pathology group, the relationship between the angular gyrus-seeded DMN and memory had an early peak in early CN, then gradually decreased in MCI before increasing again in dementia. This trajectory suggests a key role of the angular gyrus-seeded DMN metabolic deterioration in memory deficit in the asymptomatic and dementia stages of AD. In contrast, no metabolism covariance networks were related to memory in non-amyloid/non-tau and tau only groups. These observations remained robust when the analyses were performed using a larger validation cohort or the alternative ordering strategy of merging both MCI and dementia stages.DISCUSSION
The HIP structural network is identified to be associated with memory impairment in all three pathology groups which is consistent with the role that the hippocampus plays in memory cognitive domain. The trajectories of the relationship with memory suggest the hippocampal structural network integrity had an early influence of on memory performance in the preclinical AD stage and played a lesser role on memory performance as the cognitive stages progress. Our findings also suggested that the hippocampal network integrity had a more modest influence on memory in individuals without Aβ pathology compared to those with Aβ pathology. While impaired glucose uptake in the ANG is consistently shown to be an important feature for predicting memory and executive functioning performance in the later stages of AD, our present findings provide further insights into the early critical role of ANG-based metabolic covariance network for intact memory (i.e., earlier peak of beta) in the preclinical AD stage. Early malfunctioning of the ANG may predispose CN individuals with AD pathology to a more vulnerable memory system. Our hypothesis of an ANG-based metabolic compensatory mechanism in the late CN/MCI stage of AD needs to be confirmed in a larger cohort with longitudinal follow up.CONCLUSION
Our findings provide the first evidence supporting a pathology-specific non-linear relationship between structural and metabolic networks with memory across the AD continuum, highlighting potential windows of opportunity for early intervention at the preclinical AD stage to modify disease trajectory.Acknowledgements
Data collection and sharing for this project was funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. We also acknowledge the support from Yong Loo Lin School of Medicine, National University of Singapore (J.H.Z), the Duke-NUS/Khoo Bridge Funding Award (J.H.Z.), and NMRC Open Fund Large Collaborative Grant (J.H.Z.).References
1. Braak, H. and E. Braak, Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica, 1991. 82(4): p. 239-259.
2. Serrano-Pozo, A., et al., Neuropathological alterations in Alzheimer disease. Cold Spring Harbor perspectives in medicine, 2011. 1(1): p. a006189.
3. Zhou, J., et al., Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain, 2010. 133(5): p. 1352-1367.
4. Krishnan, A., et al., Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage, 2011. 56(2): p. 455-475.
5. Hong, Z., et al., Differential age-dependent associations of gray matter volume and white matter integrity with processing speed in healthy older adults. Neuroimage, 2015. 123: p. 42-50.
Figures