3125
Cerebellar iron accumulation in idiopathic cervical dystonia evaluating by quantitative susceptibility mapping1Department of Neurology & Institute of Neurology, Ruijin Hospital, affiliated with Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
Synopsis
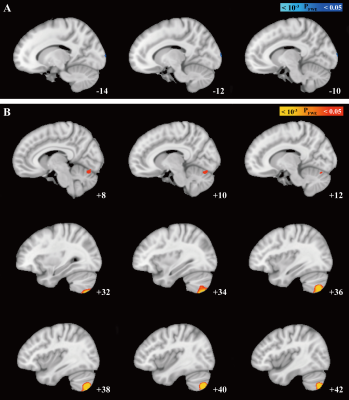
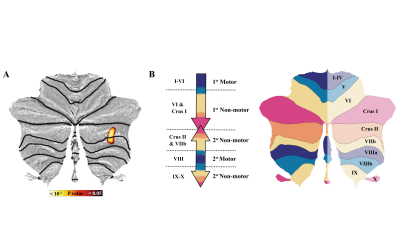
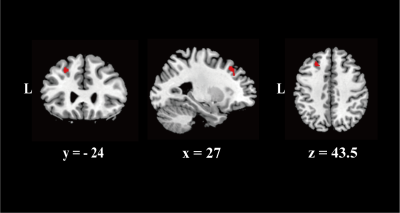
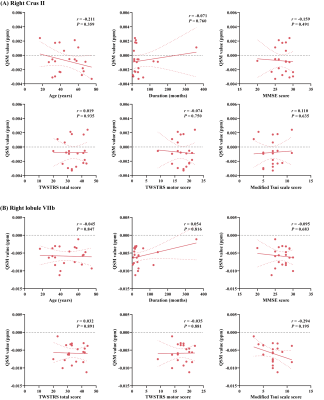
We found increased susceptibility in patients compared with HCs that primarily involved cerebellar structures . The result of 2D-flatmap visualization showed that the involved subregions of iron accumulation were in the right Crus Ⅱ and right lobule Ⅶb. Interestingly, no significant differences in QSM value of basal ganglia, thalamus, or motor cortex were observed between ICD patients and HCs. Moreover, there was no significant difference between groups regarding the GMV in the cerebellum. Of consistent findings with standard structural MRI technique highlights the potential of probing tissue iron across the cerebellum with QSM as a disease biomarker
Cervical dystonia (CD), the most common form of adult-onset focal dystonia, is characterized by involuntary muscle contractions in the neck casing twisting movement, abnormal posture, or both. The etiology of CD can be classified as inherited, acquired (known specific cause), and idiopathic (unknown cause). Purkinje cells (PCs), located in the middle layer of the cerebellar cortex, are critical for generating cerebellar output functions, which constitute the sole output for all motor coordination and learning from the cerebellar cortex and send projections to the deep cerebellar nuclei1. The evidence from the transgenic rodent models of dystonia shows abnormal PCs activities in the cerebellum2-5, which mediated dystonia3-6. Purkinje cells loss has been observed in the patients with cervical dystonia (CD)7.
Iron homeostasis is needed to maintain normal physiological brain function, whereas chronic iron overload might induce neurotoxic consequences and lead to cell death through different mechanisms (e.g., oxidative damage)8. At neuronal death, iron is released in the extracellular space where it stimulates inflammation. The distribution of non-heme iron in human cerebellar PCs has been confirmed9. Moreover, the intraventricular injections of iron induce cerebellar PCs reduction in a rodent study, which indicate that the PCs are sensitive to the damaging effect of iron10. These results, therefore, suggest that the alterations of iron deposition could be associated with the function of PCs in CD patients. While the spatial distribution of iron deposition in CD patients has not yet been elucidated.
Objectives
This study aimed to investigate whole-brain iron distribution in idiopathic CD patients using quantitative susceptibility mapping (QSM) and their clinical relevance. Methods and materials Twenty-one patients with idiopathic CD (15 females) and 21 age- and sex-comparable healthy controls (HCs) were recruited. The voxel-wise analysis was performed to access brain tissue magnetic susceptibility differences. We used the SUIT toolbox to obtain a two-dimensional (2D) representation of cerebellum, which visualized the involved subregions. Moreover, the between-group difference in the gray matter volume (GMV) was investigated.
Results
We found increased susceptibility in patients compared with HCs that primarily involved cerebellar structures (Pfwe <0.05). The result of 2D-flatmap visualization showed that the involved subregions of iron accumulation were in the right Crus Ⅱ and right lobule Ⅶb. Interestingly, there was no significant difference between groups regarding the GMV in the cerebellum.
Conclusions
Our study firstly demonstrated a spatial distribution of iron alterations in CD patients and found iron overload mainly occurred in the cerebellum. Contrarily, no significant alterations in GMV were observed with structural measurements. These results highlighted the role of cerebellum in the pathophysiology of CD and provided new insights into the behavior of QSM as a potential disease biomarker.
Acknowledgements
We would like to thank all patients for participating in this study. We thank Mrs. Xiaohui Shen for her invaluable help with the drawing. We also thank Mr. Jun Li for his kind suggestions on the analysis of imaging data.References
1. Dusart I, Flamant F. Profound morphological and functional changes of rodent Purkinje cells between the first and the second postnatal weeks: a metamorphosis? Frontiers in neuroanatomy. 2012;6:11. doi:10.3389/fnana.2012.00011
2. Liu Y, Xing H, Wilkes BJ, et al. The abnormal firing of Purkinje cells in the knockin mouse model of DYT1 dystonia. Brain research bulletin. 2020;165:14-22. doi:10.1016/j.brainresbull.2020.09.011
3. Fremont R, Tewari A, Angueyra C, Khodakhah K. A role for cerebellum in the hereditary dystonia DYT1. Elife. Feb 15 2017;6doi:10.7554/eLife.22775
4. Fremont R, Calderon DP, Maleki S, Khodakhah K. Abnormal high-frequency burst firing of cerebellar neurons in rapid-onset dystonia-parkinsonism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(35):11723-11732. doi:10.1523/JNEUROSCI.1409-14.2014
5. Fremont R, Tewari A, Khodakhah K. Aberrant Purkinje cell activity is the cause of dystonia in a shRNA-based mouse model of Rapid Onset Dystonia-Parkinsonism. Neurobiology of Disease. Oct 2015;82:200-212. doi:10.1016/j.nbd.2015.06.004
6. Raike RS, Pizoli CE, Weisz C, van den Maagdenberg AMJM, Jinnah HA, Hess EJ. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiology of disease. 2013;49:200-210. doi:10.1016/j.nbd.2012.07.019
7. Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol. Mar 2013;241:95-104. doi:10.1016/j.expneurol.2012.11.019
8. Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology. 2014;13(10):1045-1060. doi:10.1016/S1474-4422(14)70117-6
9. Korzhevskiy DE, Sukhorukova YG, Gusel'nikova VV, Kirik OV, Grigoriyev IP. [INTRANUCLEAR IRON DISTRIBUTION IN THE PURKINJE CELLS OF HUMAN CEREBELLUM]. Morfologiia. 2015;148(4):49-51.
10. Gulturk S, Kozan R, Bostanci MO, Sefil F, Bagirici F. Inhibition of neuronal nitric oxide synthase prevents iron-induced cerebellar Purkinje cell loss in the rat. Acta neurobiologiae experimentalis. 2008;68(1):26-31.
Figures