3117
Clinical variables, deep learning and radiomics features help predict the prognosis of anti-NMDA receptor encephalitis in Southwest China1Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China, 2College of Computer & Information Science, Southwest University, Chongqing, China, Chongqing, China, 3The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Synopsis
The establishment and validation of accurate prognostic models in anti-NMDA receptor (NMDAR) encephalitis is lacking. This study aims to conduct an artifificial intelligence (AI) scheme to predict the prognosis of patients with anti-NMDAR encephalitis using clinical and machine learning features. We first bulid the clinical, deep learning and radiomics models, respectively. Then, we fuse the three schemes to build a fusion model and use an independent external dataset for further validation. The new fusion model significantly outperforms all other models. It demonstrates that applying AI method is an effective way to improve the performance of prognosis prediction in anti-NMDAR encephalitis.
Body of the Abstract
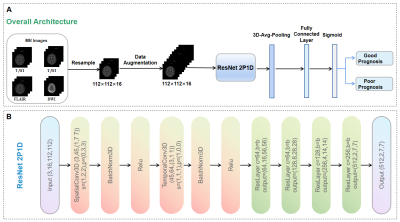
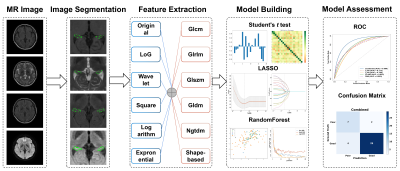
INTRODUCTION: Prospective observations of functional outcomes and establishment of prognostic prediction models are lacking in adult patients with anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis.METHODS: From January 2012, a two-center prospective study of anti-NMDAR encephalitis was initiated to collect clinical and MRI data from consecutively enrolled patients in Southwest China. Two experienced neurologists independently assess the patients’ disease severity at 2 year and the last follow-up based on the modified Rankin scale (mRS) (good outcome defined as mRS 0–2; bad outcome defined as mRS 3-5). Based on the clinical data of patients with acute anti-NMDAR encephalitis, risk factors affecting their poor outcomes were studied. Five DL and radiomics models trained with four single or combined four MRI sequences (T1-weighted imaging, T2-weighted imaging, fluid-attenuated inversion recovery imaging and diffusion weighted imaging) and a clinical model were developed to predict the prognosis of anti-NMDAR encephalitis. A fusion model combing a clinical model and two machine learning-based models was built. The performances of the fusion model, clinical model, DL-based models and radiomics-based models were compared using the area under the receiver operating characteristic curve (AUC) and accuracy and then assessed by paired t-tests (p Value < 0.05 was considered significant).
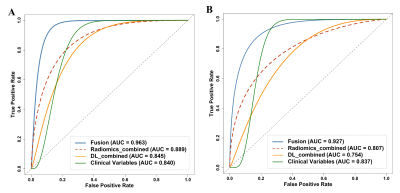
RESULTS: The fusion model achieved the significantly greatest predictive performance in the internal test dataset with an AUC of 0.963 (95% CI: [0.874-0.999]), and also significantly exhibited an equally good performance in the external validation dataset, with an AUC of 0.927 (95% CI: [0.688-0.975]) (p < 0.05). The radiomics_combined model (AUC: 0.889; accuracy: 0.857) provided significantly superior predictive performance than the DL_combined (AUC: 0.845; accuracy: 0.857) and clinical models (AUC: 0.840; accuracy: 0.905), whereas the clinical model showed significantly higher accuracy. Compared with all single-sequence models, the DL_combined model and the radiomics_combined model had significantly greater AUCs and accuracies.
DISCUSSION: This artificial intelligence scheme appears to be a promising model for anti-NMDAR encephalitis prognostic prediction with broad development prospects, potentially influencing future treatment decisions. In future, fusion of clinical, deep learning and radiomics features may have a potential to handle the classification task with limited dataset in medical imaging.
CONCLUSION: The fusion model combining clinical variables and machine learning-based models may have early predictive value for poor outcomes associated with anti-NMDAR encephalitis.
Acknowledgements
We would like to acknowledge all of the subjects who participated in this study.References
1. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391-404.
2. Warren N, Flavell J, O'Gorman C, et al. Screening for anti-NMDAR encephalitis in psychiatry. J Psychiatr Res 2020;125:28-32.
3. Gu Y, Zhong M, He L, et al. Epidemiology of Antibody-Positive Autoimmune Encephalitis in Southwest China: A Multicenter Study. Front Immunol 2019;10:2611.
4. Wang W, Li JM, Hu FY, et al. Anti-NMDA receptor encephalitis: clinical characteristics, predictors of outcome and the knowledge gap in southwest China. European Journal of Neurology 2015;23:621-629.
5. Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. The Lancet Neurology 2013;12:157-165.
6. Chi X, Wang W, Huang C, et al. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand 2017;136:298-304.
7. Broadley J, Seneviratne U, Beech P, et al. Prognosticating autoimmune encephalitis: A systematic review. J Autoimmun 2019;96:24-34.
8. Gong X, Chen C, Liu X, et al. Long-term Functional Outcomes and Relapse of Anti-NMDA Receptor Encephalitis: A Cohort Study in Western China. Neurol Neuroimmunol Neuroinflamm 2021;8.
9. Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 2019;92:e244-e252.
10. Qiu X, Zhang H, Li D, et al. Analysis of Clinical Characteristics and Poor Prognostic Predictors in Patients With an Initial Diagnosis of Autoimmune Encephalitis. Front Immunol 2019;10:1286.
11. Dubey D, Samudra N, Gupta P, et al. Retrospective case series of the clinical features, management and outcomes of patients with autoimmune epilepsy. Seizure 2015;29:143-147.
12. Heine J, Pruss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis--Relevance for clinical practice and hippocampal function. Neuroscience 2015;309:68-83.
13. Kelley BP, Patel SC, Marin HL, Corrigan JJ, Mitsias PD, Griffith B. Autoimmune Encephalitis: Pathophysiology and Imaging Review of an Overlooked Diagnosis. AJNR Am J Neuroradiol 2017;38:1070-1078.
14. Hu Q, Whitney HM, Giger ML. A deep learning methodology for improved breast cancer diagnosis using multiparametric MRI. Sci Rep 2020;10:10536.
15. Xu Y, He X, Li Y, Pang P, Shu Z, Gong X. The Nomogram of MRI-based Radiomics with Complementary Visual Features by Machine Learning Improves Stratification of Glioblastoma Patients: A Multicenter Study. J Magn Reson Imaging 2021;54:571-583.
16. Truhn D, Schrading S, Haarburger C, Schneider H, Merhof D, Kuhl C. Radiomic versus Convolutional Neural Networks Analysis for Classification of Contrast-enhancing Lesions at Multiparametric Breast MRI. Radiology 2019;290:290-297.
17. Wu W, Li J, Ye J, Wang Q, Zhang W, Xu S. Differentiation of Glioma Mimicking Encephalitis and Encephalitis Using Multiparametric MR-Based Deep Learning. Front Oncol 2021;11:639062.
18. Yin P, Mao N, Zhao C, Wu J, Chen L, Hong N. A Triple-Classification Radiomics Model for the Differentiation of Primary Chordoma, Giant Cell Tumor, and Metastatic Tumor of Sacrum Based on T2-Weighted and Contrast-Enhanced T1-Weighted MRI. J Magn Reson Imaging 2019;49:752-759.
19. Bron EE, Klein S, Papma JM, et al. Cross-cohort generalizability of deep and conventional machine learning for MRI-based diagnosis and prediction of Alzheimer's disease. Neuroimage Clin 2021;31:102712.
20. Sacca V, Sarica A, Novellino F, et al. Evaluation of machine learning algorithms performance for the prediction of early multiple sclerosis from resting-state FMRI connectivity data. Brain Imaging Behav 2019;13:1103-1114.
21. Xiang Y, Zeng C, Liu B, et al. Deep Learning-Enabled Identification of Autoimmune Encephalitis on 3D Multi-Sequence MRI. J Magn Reson Imaging 2021.
22. Du T, Heng W, Lorenzo T, et al. A Closer Look at Spatiotemporal Convolutions for Action Recognition. 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition 2018:6450-6459.
23. Wang JL, Jiao JB, Bao IC, et al. Self-supervised Video Representation Learning by Uncovering Spatio-temporal Statistics. IEEE Trans Pattern Anal Mach Intell 2021.
24. Aerts HJ, Velazquez ER, Leijenaar RT, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014;5:1-9.
25. Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 2017;14:749-762.
26. L Y. Awareness and Cooperation in Neural Network Ensemble Learning. 2019 12th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI) 2019:1-5.
27. Davies G, Irani SR, Coltart C, et al. Anti-N-methyl-D-aspartate receptor antibodies: A potentially treatable cause of encephalitis in the intensive care unit. Critical Care Medicine 2010;38:679-682.
28. Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Current Opinion in Neurology 2014;27:361-368.
29. Zhang W, Fang M, Dong D, et al. Development and validation of a CT-based radiomic nomogram for preoperative prediction of early recurrence in advanced gastric cancer. Radiotherapy and Oncology 2020;145:13-20.
30. Li J, Dong D, Fang M, et al. Dual-energy CT–based deep learning radiomics can improve lymph node metastasis risk prediction for gastric cancer. European Radiology 2020;30:2324-2333.
31. Yin P, Mao N, Liu X, et al. Can clinical radiomics nomogram based on 3D multiparametric MRI features and clinical characteristics estimate early recurrence of pelvic chondrosarcoma? Journal of Magnetic Resonance Imaging 2019;51:435-445.
32. Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain 2012;135:1622-1638.
33. Finke C, Kopp UA, Scheel M, et al. Functional and structural brain changes in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2013;74:284-296.
34. Finke C, Kopp UA, Pajkert A, et al. Structural Hippocampal Damage Following Anti-N-Methyl-D-Aspartate Receptor Encephalitis. Biol Psychiatry 2016;79:727-734.
35. Lo Gullo R, Eskreis-Winkler S, Morris EA, Pinker K. Machine learning with multiparametric magnetic resonance imaging of the breast for early prediction of response to neoadjuvant chemotherapy. Breast 2020;49:115-122.
36. Yuan Y, Qin W, Buyyounouski M, et al. Prostate cancer classification with multiparametric MRI transfer learning model. Med Phys 2019;46:756-765.
37. Xia X, Gong J, Hao W, et al. Comparison and Fusion of Deep Learning and Radiomics Features of Ground-Glass Nodules to Predict the Invasiveness Risk of Stage-I Lung Adenocarcinomas in CT Scan. Front Oncol 2020;10:418.
38. Sun Q, Lin X, Zhao Y, et al. Deep Learning vs. Radiomics for Predicting Axillary Lymph Node Metastasis of Breast Cancer Using Ultrasound Images: Don't Forget the Peritumoral Region. Front Oncol 2020;10:53.
Figures