3111
Intravoxel incoherent motion imaging of masticatory muscles in patients with head and neck cancer
Chia-Wei Lin1, Kai-Lun Cheng2, Hsueh-Ju Lu3, Ying-Hsiang Chou1,4, Yeu-Sheng Tyan1,2, and Ping-Huei Tsai1,2
1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan, 3Division of Medical Oncology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan, 4Department of Radiation Oncology, Chung Shan Medical University Hospital, Taichung, Taiwan
1Department of Medical Imaging and Radiological Sciences, Chung Shan Medical University, Taichung, Taiwan, 2Department of Medical Imaging, Chung Shan Medical University Hospital, Taichung, Taiwan, 3Division of Medical Oncology, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan, 4Department of Radiation Oncology, Chung Shan Medical University Hospital, Taichung, Taiwan
Synopsis
Head and Neck Cancer (HNC) is one of the most common cancers worldwide. Although skeletal muscle depletion or muscle dysfunction is associated with cancer progression, changes in masticatory muscle diffusion and perfusion in HNC are still unclear. This study aims to assess the masticatory muscle perfusion and diffusion in HNC using intravoxel incoherent motion (IVIM) imaging. Our preliminary result indicated the normalized D* values in masticatory muscles were significantly lower in HNC patients than that in controls, suggesting the possibility of reduced masticatory muscle perfusion in HNC.
Introduction
Head and Neck Cancer (HNC) is one of the most common cancers worldwide, which could be associated with the consumption of alcohol, tobacco and betel nuts. Sarcopenia, defined as skeletal muscle depletion, is an independent adverse prognostic factor for overall survival (OS) and disease-free survival (DFS) in HNC patients, especially in patients with worse performance or locally advanced disease [1]. Masticatory muscle mass is a potential indicator of nutritional status, physical activity level, and trauma-related prognosis. While the pathophysiological mechanism is not well-known, deterioration in masticatory muscle quality could play an important role in patients with HNC [2]. Moreover, vascular impairments, such as lower muscle capillarization, may contribute to declines in muscle mass and function due to lack of nutrients and oxygen from the reduced blood flow [3]. Previous studies have demonstrated the feasibility of intravoxel incoherent motion imaging (IVIM) for quantifying perfusion and diffusion effects in skeletal muscles at rest and after exercise [4]. Therefore, this study aims to assess the masticatory muscle perfusion and diffusion in patients with HNC and controls using IVIM scheme.Methods
A total of 26 patients with HNC and 16 age-matched controls were included in thisstudy. MRI examinations were performed at a 3T MR scanner (MagnetomSkyra, Siemens Healthcare, Erlangen, Germany) with a 20-channel head and neck coil. In addition to the conventional imaging protocols, a multishot readout-segmented EPI with fat suppression was acquired with: TR/TE=4300ms/64ms, FOV=230x230mm², matrix size=160*160, slice thickness=5mm, number of slice=32, iPAT= 2, bandwidth = 919Hz/Px, b value=0, 50, 200, 400, 600, 800s/mm². All images in our study have been realigned (estimate & reslice) first, and then IVIM-related parameters (D: diffusion coefficient, D*: peudodiffusion coefficient, and f: perfusion fraction) were calculated by fitting the biexponential MR signal attenuation curve using the DWI images of the corresponding b-values. Manual ROIs selection was performed on DWI images, using T1 images as reference, covering three masticatory muscles: masseter muscle (MM), lateral pterygoid muscle (LPM), and medial pterygoid muscle (MPM) (Figure 1). Afterwards, the derived IVIM-related parameters were normalized by the mean values in medulla oblongata to obtain the muscle-to-medulla ratio of the parameters. Finally, the intraclass correlation coefficient (ICC) was used to assess the intra-observer variability and a nonparametric Mann–Whitney U test was used to compare the differences of the parameters between HNC patients and controls.Results
The derived ICC values were shown in Table 1. The intraobserver agreement was excellent for D, D*, and f of the bilateral masticatory muscles, respectively (all above 0.9). DWI images and the corresponding IVIM-related parametric maps, including D, D* and f value, covering the bilateral masseter muscles of a normal control and a HNC patient were illustrated in Figure 2. Comparisons of the masticatory muscle perfusion and diffusion between the HNC patients and normal controls were shown in Figure 3 (right side) and Figure 4 (left side), respectively. While no significant differences of the normalized D and f values in the three masticatory muscles were found between the two groups (p > 0.05), the normalized D* values in the muscles (except in the right MM) were significantly lower in HNC patients than that in controls (p < 0.05).Disccusion
Our preliminary study demonstrated the feasibility of IVIM imaging to evaluate the masticatory muscle perfusion and diffusion in patient with HNC. While the cause of the cancer-related muscle dysfunction is complex, the normalized D* values in the masticatory muscles were significantly lower in HNC patients than that in controls, suggestive of the possibility of reduced masticatory blood flow during cancer progression. This result is in coincidence with previous reports [5]. Additionally, no significant differences of water diffusion in the masticatory muscles were obtained between the two groups, probably resulting from the averaging effect of the trace DWI signals. Investigation on changes of the directional water diffusion in masticatory muscles may contribute to further understanding its pathophysiological mechanism. In conclusion, this study indicates the important role of IVIM imaging in assessing the masticatory muscle perfusion and diffusion in patients with HNC. The normalized D* values could have potential to reveal the information of altered masticatory muscle capillarization, which may provide an alternative for further prognosis prediction in HNC.Acknowledgements
This study was supported by the Ministry of Science and Technology, Taipei, Taiwan (MOST 108-2221-E-040-007-MY3).References
1.van Rijn-Dekker MI, et al. Impact of sarcopenia on survival and late toxicity in head and neck cancer patients treated with radiotherapy.RadiotherOncol. 2020;147:103-110 2.Change SW, et al. Masticatory muscle index for indicating skeletal muscle mass in patients with head and neck cancer. PLoS One 2021;16(5):e0251455. 3.Prior SJ, et al. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J Gerontol A Bio Sci Med Sci. 2016;71(8):1096-101 4.Filli L, et al. Dynamic intravoxel incoherent motion imaging of skeletal muscle at rest and after exercise.NMR Biomed. 2015;28(2):240-6 5. Christensen JF, et al. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25:947-58Figures
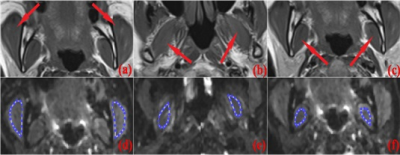
Fig. 1 Illustration of ROIs
selection. (a-c) are T1-weighted images covering the three masticatory muscles:
masseter muscle (MM), lateral pterygoid muscle (LPM),
and medial pterygoid muscle (MPM), respectively. The bilateral regions of MM (d), LPM (e), and MPM (f) are manually drawn on the DWI
images.
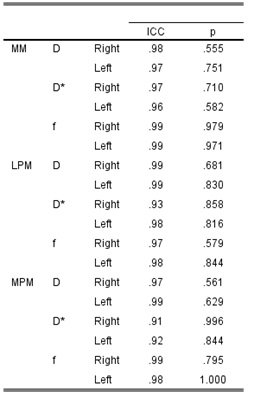
Table.1 The intraclass
correlation coefficient (ICC) of the IVIM-related parameters in the bilateral
masticatory muscles, including MM, LPM, and MPM.
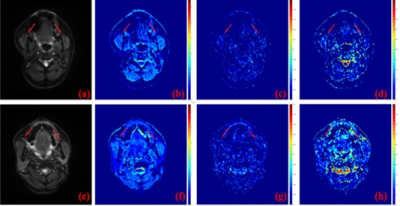
Fig. 2 DWI images and the derived
IVIM-related parametric maps, including D map, D* map, and f map, of a normal
control (a-d) and a patient with HNC (e-h) are shown, respectively.
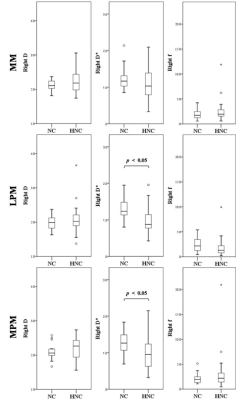
Fig.3 Comparisons
of the perfusion and diffusion effects in the right masticatory muscles (MM,
LPM, and MPM) between the HNC patients (HNC) and normal
controls (NC).
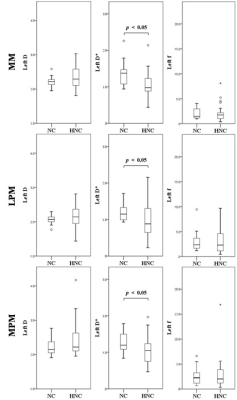
Fig.4 Comparisons
of the perfusion and diffusion effects in the left masticatory muscles (MM,
LPM, and MPM) between the HNC patients (HNC) and normal
controls (NC).
DOI: https://doi.org/10.58530/2022/3111