3103
Differential Diagnosis of Dermatofibrosarcoma Protuberans: Clinical Values of High-resolution DCE-MRI1Radiology, The First Affiliated Hospital of Soochow University, SUZHOU, China, 2Dermatology, The First Affiliated Hospital of Soochow University, SUZHOU, China, 3Philips Healthcare, SHANGHAI, China, 4Radiology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, HANGZHOU, China
Synopsis
Dermatofibrosarcoma protuberans (DFSP) is a type of intermediately malignant cutaneous spindle cell neoplasms, which is easy to be confused with several benign ones even after needle biopsy, especially cellular fibrous histiocytoma (cFH), resulting in inadequate excision and local recurrence. We found that as a novel skin imaging technique, high-resolution (HR) DCE MRI could distinguish DFSP. The features include infiltration of surrounding fat, ill-defined margins and large quantitative parameters. Both DFSP and cFH have type-III time-signal intensity curves (TICs). In contrast, other confused lesions presented type-II-or-I TIC. The recommendation of preoperative HR-MRI could assist dermatologists to perform surgical plan more confidently.
Introduction
DFSP is an uncommon cutaneous fibrohistiocytic neoplasm, which accounts for 1% of all soft tissue sarcomas, with an incidence of 0.8 to 5 cases per million population each year1. Typically, DFSP presents slowly progressive, painless cutaneous protuberance that may begin as a pink plaque. It is an intermediately malignant (rarely metastasizing) cutaneous spindle cell neoplasm2, presenting diffuse spindle cells arranged in a storiform pattern under light microscopes. The tumor cells infiltrate subcutaneous fat in a honeycomb pattern3. CD34 is usually positively expressed while factor XIII-a and CD68 are negative1. DFSP is notable for its high local recurrence after surgical resection. The recurrent lesions are prone to sarcomatous transformation and metastasis4 peculiarly. Wide local excision (WLE) is recommended to decrease the recurrence rates, and 2 to 3 cm margins are considered to be appropriate5 generally. The determination of invasion layers is crucial especially when tumors invade the underlying fascia or muscle. DFSP is easy to be confused with several benign spindle cell neoplasms, including fibrous histiocytoma (FH, also known as dermatofibroma) especially cellular variants (cFH), keloids and nodular fasciitis (NF). The aim of this study was to explore clinical values of dynamic contrast-enhanced (DCE) HR-MRI in differential diagnosis of DFSP.Methods
Subjects: A total of 28 patients with clinical suspicion of DFSP (15 males, age range: 6-69 years) were enrolled.Acquisition Protocol: All examinations were performed using a dedicated 7 centimeter-diameter single-element surface coil with 32 receiver channels on a 3T MRI scanner. The coil was placed as close to the lesions as possible, meanwhile keeping the lesion as central as possible6. High-resolution non-contrast T1-and-T2-weighted images (T1WI and T2WI) were acquired firstly. Parameters were as follows: T1WI, repetition time (TR) 612 microsecond (ms), echo time (TE) 14ms, 1 time signal averaging, field of view (FOV) 80*80 mm2, matrix 320*320, section thickness 1-5 mm, turbo factor 3, voxel size 0.3*0.3*2.0 mm3. T2WI, TR 4790 ms, TE 96 ms, 2 signal averaging, FOV 80*80 mm2, matrix 384*384, section thickness 1-5 mm, turbo factor 6, voxel size 0.2*0.2*2.0 mm3. Bi-flip-angle T1 mapping sequences were performed prior to DCE scanning, with a flip angle of 2° and 15°. DCE-MRI was performed after intravenous administration of 0.2 mmol/kg gadopentetate dimeglumine (Gd-DTPA) at a rate of 3 mL/s. 36 phases were collected. The relevant scanning parameters were as follows: TR 4.21ms, TE 1.42ms, 1 signal averaging, FOV 142*142 mm2, matrix 256*256, section thickness 3 mm, voxel size 0.6*0.6*3.0 mm3.
Data Processing: Two radiologists who were blinded to all histopathological results evaluated HR-MRI features of all cases independently. Consensus was reached through consultation in case of any disagreement. Evaluated features included T1 and T2 signal, depth, major diameter, morphology, margin, cutaneous involvement layer and enhancement features. One radiologist, who was also blinded to the pathological data, drew oval regions of interest (ROIs) on the images using commercial post-processing software called Tissue 4D. 1-2 cm2 ROIs were placed several times in homogeneous enhancement regions as large as possible. Quantitative parameters of ROIs were automatically generated based on a modified hemodynamic two-compartment Tofts model, including Ktrans, Kep, Ve and iAUC. MeanCurve, a commercial software, was used to generate TICs.
Analysis: The continuous variables were given as mean ± standard deviation. All data analysis was performed using SPSS 23.0. Fisher exact test was used to analyze whether TIC types were different between groups. One way ANOVA was used to compare quantitative parameters of DFSP, FH and keloid. The difference was statistically significant when P <0.05.
Results
7 cases of DFSP, 9 cases of FH (4 cFH), 11 cases of keloid and 1 case of NF were collected. DFSP presented relatively high T2WI signal, ill-defined margins, irregular morphology and infiltration of surrounding fat (signs included tiny spinous protrusion, crab-foot-like spicules of the basal, satellite lesions and broken capsule (Figure 1). TIC types between lesions were significantly different. Most of DFSP and cFH had type-III TIC (10 cases out of 11 cases). The comparison of quantitative perfusion parameters is shown in Table 1, with DFSP having the highest values. Of all cases, HR-MRI displayed that 1 case involved muscle, 5 cases involved deep fascia, 11 cases involved subcutis, and 11 cases involved dermis. All patients undergoing preoperative HR-MR got pathologically negative basal margin after the first operation.Discussion
Due to its high-resolution presentation, HR-MRI could clearly reveal the infiltration of surrounding fat in DFPS, which coincided with the honeycomb-pattern infiltration of spindle cells into subcutaneous fat in pathology. This is the most compelling evidence that supports DFSP diagnosis. CFH had vague margins in some cases but no fat infiltration signs (Figure 2). Both cFH and DFSP have theoretical risk of local recurrence7, similar necessity for WLE (cFH needs 1-2cm margins )8, and type-III TIC, which means type-III TIC might suggest potential recurrence risk and regular follow-up after operation should be emphasized. Quantitative perfusion parameters might help in the identification of DFSP. When it comes to operation planning, invasion layers determined by HR-MRI are reliable.Conclusion
HR-MRI is a reliable imaging technique in differentiating DFSP from other confusing cutaneous spindle cell neoplasms.Acknowledgements
This work was mainly supported by the program for Gusu Medical talent of Suzhou city(grant number GSWS2020009, the Translational Research Grant of NCRCH(grant number2020WSB06, National Natural Science Foundation of China (grant number 81671743), the clinical key diseases diagnosis and therapy special project of Health and Family Planning Commission of Suzhou (LCZX201801), the program for Advanced Talents within Six Industries of Jiangsu province (WSW-057), and the High-level Health Personnel “six-one” Project of Jiangsu province in China (LGY2016035).References
1. Allen A, Ahn C, Sangüeza OP. Dermatofibrosarcoma Protuberans. Dermatol Clin. 2019;37(4):483-488.
2. Choi JH, Ro JY. The 2020 WHO Classification of Tumors of Soft Tissue: Selected Changes and New Entities. Adv Anat Pathol. 2021;28(1):44-58.
3. Choi JH, Ro JY. Cutaneous Spindle Cell Neoplasms: Pattern-Based Diagnostic Approach. Arch Pathol Lab Med. 2018;142(8):958-972.
4. Grizzetti L, Gaide O, Kuonen F. Dermatofibrosarcoma protuberans : clinico-pathological aspects and management. Rev Med Suisse. 2020;16(688):640-645.
5. Acosta AE, Santa Ve´lez C. Dermatofibrosarcoma protuberans. Curr Treat Options Oncol. 2017;18(9):56.
6. Budak MJ, Weir-McCall JR, Yeap PM, et al. High-Resolution Microscopy-Coil MR Imaging of Skin Tumors: Techniques and Novel Clinical Applications. Radiographics. 2015;35(4):1077-1090.
7. Hornick JL. Cutaneous soft tissue tumors: how do we make sense of fibrous and "fibrohistiocytic" tumors with confusing names and similar appearances?. Mod Pathol. 2020;33(Suppl 1):56-65.
8. Berklite L, Ranganathan S, John I, Picarsic J, Santoro L, Alaggio R. Fibrous histiocytoma/dermatofibroma in children: the same as adults?. Hum Pathol. 2020;99:107-115.
Figures
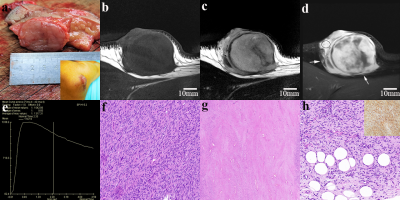
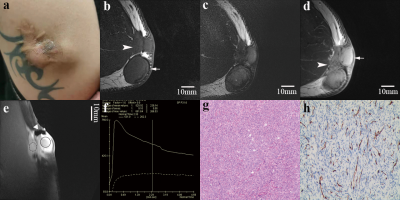
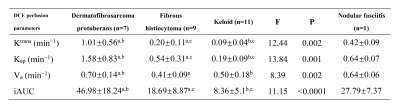
Table 1 Quantitative perfusion parameters of DCE-MRI
Note: Data are presented as means ± standard deviations. The same “a” or “b” or “c” at the top-right corner within the same line means there is significantly difference between both (p<0.05).