3089
The potential role of MRI-based bone marrow Radiomics in survival prediction of newly diagnosed multiple myeloma1Peking university people's hospital, Beijing, China
Synopsis
Multiple myeloma (MM) is the second most common hematologic malignancy. Despite the more effective therapies were introduced, this incurable disease remains highly heterogeneous in clinical outcome. Accurate predicative markers for prognosis are needed to develop appropriate treatment in newly diagnosed MM.
Purpose
To explore the potential clinical value of MRI-based bone marrow Radiomics in predicting survival in multiple myeloma (MM) patients.Material and methods
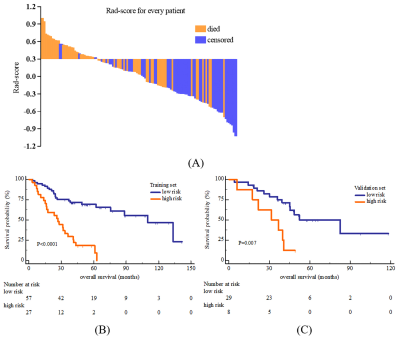
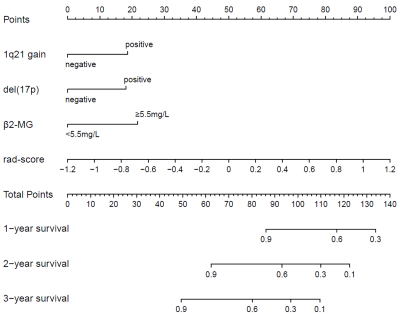
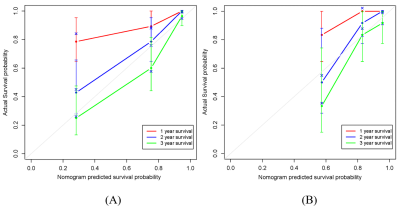
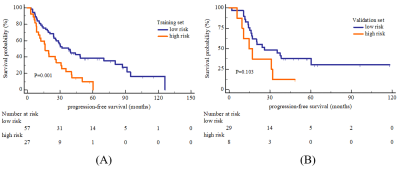
A total of 121 MM patients was enrolled and divided into training (n=84) and validation (n=37) sets. The radiomics features were extracted from lumbar MRI and selected by the least absolute shrinkage and selection operator (LASSO) algorithm to develop a radiomics score (Rad-score). The multivariate cox analysis was used for constructing radiomics nomogram for MM overall survival (OS) prediction. The predictive ability and accuracy of the nomogram were evaluated by the index of concordance (C-index) and calibration curves, and compared with other 4 models including the clinical model, Rad-score model, the Durie-Salmon staging system (D-S) and the International Staging System (ISS). The potential association between OS-based radiomics and progression-free survival (PFS) was also explored.Results
The high and low risk group stratified by Rad-score showed significant different OS in both training (log-rank P<0.0001) and validation set (log-rank P=0.007). The predictive ability of radiomics nomogram incorporated the Rad-score and independent clinical risk factors (1q21 gain, del (17p), and β2-MG≥5.5 mg/L) was better than the other 4 models (C-index: 0.793 vs.0.733 vs.0.742 vs.0.554 vs.0.671 in training set, and 0.812 vs.0.799 vs.0.717 vs.0.512 vs.0.761 in validation set). The OS-based radiomics lacked the predictive ability for PFS (log-rank P=0.001 in training set and log-rank P=0.103 in validation set), whereas the 2-year and 3-year PFS showed significant difference between the high and low risk groups stratified by OS-based Rad-score (0.4 vs.0.628, P=0.02 and 0.2 vs.0.488, P=0.003, respectively).Conclusion
The MRI-based bone marrow Radiomics was a valuable additional tool for OS prediction, and OS-based radiomics had certain association with PFS.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No.81971575).References
1. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol (2014) 15(12):e538-48. doi: 10.1016/S1470-2045(14)70442-5
2. Smith D, Yong K. Advances in understanding prognosis in myeloma. Br J Haematol (2016) 175(3):367-80. doi: 10.1111/bjh.14304.
3. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia (2009) 23(1):3-9. doi: 10.1038/leu.2008.291
4. Matsue K, Kobayashi H, Matsue Y, Abe Y, Narita K, Kitadate A, et al. Prognostic significance of bone marrow abnormalities in the appendicular skeleton of patients with multiple myeloma. Blood Adv (2018) 2(9):1032-9. doi: 10.1182/bloodadvances.2017014720
5. Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol (2015) 33(6):657-64. doi: 10.1200/JCO.2014.57.9961
6. Moulopoulos LA, Dimopoulos MA, Christoulas D, Kastritis E, Anagnostou D, Koureas A, et al. Diffuse MRI marrow pattern correlates with increased angiogenesis, advanced disease features and poor prognosis in newly diagnosed myeloma treated with novel agents. Leukemia (2010) 24(6):1206-12. doi: 10.1038/leu.2010.70
7. Tomaszewski MR, Gillies RJ. The Biological Meaning of Radiomic Features. Radiology (2021) 298(3):505-16. doi: 10.1148/radiol.2021202553
Figures