3084
Repeatability and feasibility of amide proton transfer imaging in musculoskeletal tumors1The Third Hospital, Hebei Medical University, Shijiazhuang, China, 2Philips healthcare,Beijing,China, Beijing, China
Synopsis
Amide proton transfer (APT) imaging is a noninvasive emerging molecular MRI technique. Currently, APT was mainly used for the diagnosis, classify and recurrence prediction of head glioma. The application in other tissue such as musculoskeletal disease is limited. The feasibility and the repeatability of APT imaging in body or other tissues is necessary for its clinical practice. In this study, we explorer the scan-rescan repeatability of APT imaging in musculoskeletal (MSK) tumor on a clinical 3.0T MR scanner, and further to differentiate malignant from benign solid tumors in the study.
Synposis
Amide proton transfer (APT) imaging is a noninvasive emerging molecular MRI technique. Currently, APT was mainly used for the diagnosis, classify and recurrence prediction of head glioma. The application in other tissue such as musculoskeletal disease is limited. The feasibility and the repeatability of APT imaging in body or other tissues is necessary for its clinical practice. In this study, we explored the scan-rescan repeatability of APT imaging in musculoskeletal (MSK) tumor on a clinical 3.0T MR scanner, and further to differentiate malignant from benign solid tumors in the study.Summary of Main Findings
The excellent scan-rescan repeatability of APT signal intensity in MSK tumors was found. Higher APT signal intensity in malignant solid MSK tumors was measured compared with those in benign MSK tumors.Introduction
Amide proton transfer (APT) imaging is sensitive to detect the small variations in amide proton concentrations associated with tumor proteins/peptide backbone1, 2. APT imaging could help to differentiate malignant from benign tumors, such as brain tumor3, 4, breast tumor5, head and neck cancer6, ovarian cystic lesion7, cervical carcinoma8, rectal cancer9 and so on in the literatures. However, as we know, there are few reports of APT imaging in MSK tumors. The study aimed to explore the repeatability of APT imaging in the MSK tumors and feasibility to distinguish benign from malignant solid MSK tumors.Method
29 patients with MSK tumor (19 malignant, 10 benign) were included in this study. There were totally 26 solid tumors and 3 cystic tumors, and all of the cystic tumors were benign. APT imaging was conducted at 3.0 T MR scanner (Ingenia CX, Philips Healthcare) with a saturation duration of 1s and B1 amplitude power of 2uT. All patients underwent twice APT imaging scans on the same day. After completing the first scan, the second scan was performed with a new reposition in the same slicer after a new survey scan. APT signal intensity (SI) were measured by two radiologists on a post-processing workstation (ISP 9, Philips Healthcare). After fusing APT images on T2-weighted images, ROIs were drawn manually to include the whole tumor on the maximum axial plane. The differences of APT SIs between the repeat scans for each patient were calculated. The normality of APT SIs was measured by the Shapiro-Wilk test. A Student’s t-test or the Wilcoxon Signed-rank test was used to test group difference of APT SI in tumors between repeat scans according to whether the normal distribution exit or not. Meanwhile, the group difference of APT SI between benign and malignant solid MSK tumors was test with a Student’s t-test or the Mann-Whitney test2, 10. The repeatability of APT SI in MSK tumors was also evaluated with Intraclass correlation coefficient (ICC), Pearson correlation coefficient, repeatability value and Bland-Altman plot. Repeatability value , which indicated the difference of APT SI between two scans greater than the repeatability value would be significant in an individual2, was calculated according to Galbraith’s method11 .Result
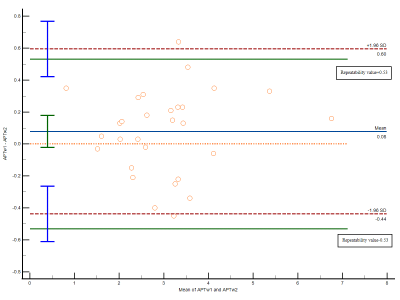
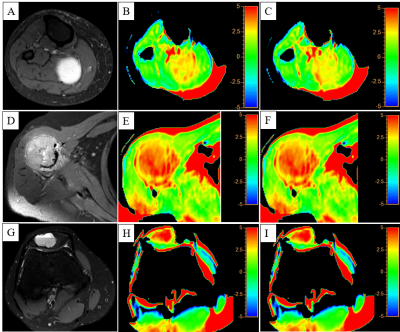
The ICC and Pearson correlation coefficient for APT SIs of two scans were 0.96 and 0.97, respectively. The repeatability value of APT SIs was 0.53%. Bland-Altman plot showed most of differences were located in the 95% limits of agreement (Figure 1). There was no significant difference in APT SIs between two scans in 29 MSK tumors (p>0.05). For 26 solid MSK tumors, the higher APT SI of malignant tumors was found compared with those of benign tumors (3.11%±1.06% vs. 2.17%±0.87%, p < 0.05). The APT value of cystic tumors were 4.41%±0.69%. Figure 2 showed the images of T2-weighted and APT imaging from a benign, a malignant and a cystic tumor.Discussion
In this study, the repeatability of APT SI in MSK tumors was excellent with the ICC, Pearson correlation coefficient and Bland-Altman plot, while it was poor in a metastatic tumor and an osteosarcoma. The two tumors were extremely inhomogeneous on T2-weighted images, which might led to the B0 inhomogeneity. However, APT imaging is very sensitive to the magnetic field inhomogeneous12. The high APT SIs in three cystic tumors were found, which may interface the accuracy of identification from malignant tumors by APT imaging. Therefore, the APT SI could only be used to diagnosis between benign and malignant solid MSK tumors. The higher APT SI in solid malignant tumors was found and this finding is consistent with that of previous study13.Conclusion
The repeatability of APT SI in MSK tumors is excellent in this preliminary study, suggesting its potential clinical application in diagnosis and efficacy evaluation.Acknowledgements
The authors wish to thank the Hebei Medical University, the Third Hospital of Hebei Medical University, and Philips Healthcare.References
1. Zhou J, Payen, Jean-Francois, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085-1090.
2. Yuan J, Chen S, AD K, et al. Amide proton transfer-weighted imaging of the head and neck at 3T: a feasibility study on healthy human subjects and patients with head and neck cancer. NMR Biomed. 2014;27(10):1239-1247.
3. Park JE, Kim HS, Park KJ, et al. Histogram Analysis of Amide Proton Transfer Imaging to Identify Contrast-enhancing Low-Grade Brain Tumor That Mimics High-Grade Tumor: Increased Accuracy of MR Perfusion. Radiol. 2015;277(1):151-161.
4. Togao O, Hiwatashi A, Yamashita K, et al. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. Eur Radiol. 2017;27(2):578-588.
5. Meng N, Wang X, Sun J, et al. Comparative Study of Amide Proton Transfer¦eighted Imaging and Intravoxel Incoherent Motion Imaging in Breast Cancer Diagnosis and Evaluation. J Magn Reson Imaging. 2020;52(4):1175-1186.
6. Lu Y, Li C, Luo X, et al. Differentiation of Malignant and Benign Head and Neck Tumors with Amide Proton Transfer-Weighted MR Imaging. Mol Imaging Biol. 2019;21(2):348-355.
7. Ishimatsu K, Nishie A, Takayama Y, et al. Amide proton transfer imaging for differentiating benign ovarian cystic lesions: Potential of first time right. Eur J Radiol. 2019;120:108656.
8. Li B, Sun H, Zhang S, et al. Amide proton transfer imaging to evaluate the grading of squamous cell carcinoma of the cervix: A comparative study using 18F FDG PET. J Magn Reson Imaging. 2019;50(1):261-268.
9. Nishie A, Takayama Y, Asayama Y, et al. Amide proton transfer imaging can predict tumor grade in rectal cancer. Magnetic Resonance Imaging. 2018;51:96-103.
10. Dula AN, Arlinghaus LR, Dortch RD, et al. Amide proton transfer imaging of the breast at 3 T: Establishing reproducibility and possible feasibility assessing chemotherapy response. Magn Reson Med. 2013;70(1):216-224.
11. Galbraith SM, Lodge MA, Taylor NJ, et al. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002;15(2):132-142.
12. Zhou J, Blakeley JO, Hua J, et al. Practical data acquisition method for human brain tumor amide proton transfer (APT) imaging. Magnetic Resonance in Medicine. 2008;60(4):842-849.
13. Kamitani T, Sagiyama K, Togao O, et al. Amide proton transfer (APT) imaging of parotid tumors: Differentiation of malignant and benign tumors. Eur J Radiol. 2020;129:109047.
Figures