3083
3D texture analysis of MRI relaxation time maps for assessment of repair cartilage with treatment of mesenchymal progenitor cells1Department of Radiology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, No. 160, Pujian Road, Shanghai, 200127, China., Shanghai, China, 2Department of Rheumatology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, No. 160, Pujian Road, Shanghai, 200127, China., Shanghai, China, 3Cellular Biomedicine Group, Inc., No. 85 Faladi Road, Building 3, Zhangjiang, Pudong New Area, Shanghai, 201210, China., Shanghai, China
Synopsis
We used 3D texture analysis of MRI relaxation times, combined with clinical outcomes, to evaluate the potential of repair cartilage with allogeneic human adipose-derived mesenchymal progenitor cells in patients with KOA. Significant differences were observed in texture RLM parameters of T1rho and T2 maps in patients. We also found correlations between WOMAC pain scores and texture parameters, suggesting the spatial heterogeneity of relaxation time maps maybe associated with clinical scores. As conclusion, texture analysis has potential applications in understanding mechanism of stem cells repairing cartilage and assessing response to treatment.
Introduction
Recent studies have suggested that assessing MR relaxation time constant maps spatial and laminar distribution could be more sensitive than full-thickness mean relaxation time values in detecting the compositional variation of cartilage1-3. We used textural analysis matrix to examine the spatial distribution of pixel values and detect the compositional variation of repair cartilage with treatment of allogeneic human adipose-derived mesenchymal progenitor cells (haMPCs).Methods
Eighteen patients participated a phase I/IIa clinical trial. All patients were divided randomly into three groups with intra-articular injections of haMPCs: the low-dose (1.0×107 cells), mid-dose (2.0×107), and high-dose (5.0×107) group with six patients each. Compositional MRI relaxation time maps including T2, T2 star and R2star and T1rho, were performed at 1 day before first injection to collect the base time point and 48 weeks to collect terminal point. The details of haMPC preparation and MRI examination were described in a previous report4. Regions of interest (ROIs) were segmented manually on fat suppressed T2-weighted images, more obvious image of cartilage boundary, using MRIcro software by two researchers who were blinded to subject age, disease status, and demographics. Six ROIs were defined as shown in Figure 1. Voxels at the tissue boundaries were also excluded. To maintain quantitative accuracy, the ROIs drawn on the R2star images were applied to relaxation time constant maps. 3D texture analyses based run-length matrix (RLM) of the segmented ROIs were conducted using MaZda software as MacKay’s report3. Five RLM parameters were analyzed, including run length non-uniformity (RLNonUni), grey level non-uniformity (GLevNonU), long run emphasis (LngREmph), short run emphasis (ShrtREmp) and fraction of image in runs (Fraction). The mean value of each RLM parameter for each pixel in all possible directions and pixel offsets was calculated for each coronal image. We used the difference before and after treatment (D-values) as the object to avoid errors caused by individual differences. WOMAC pain clinical evaluation was also conducted at the baseline and terminal points.Results
Compared with base time, there were significant differences among three dose groups in RLNonUni of T2 map (F = 9.24, P = 0.008), LngREmph of T2 map (F = 6.31, P = 0.025) and ShrtREmp of Trho map (F = 4.52, P = 0.033), whereas no significant differences in other measurements (Fig. 2). Figure 3 shows that correlations between WOMAC pain scores and texture parameters including RLNonUni of Trho map (R2 = 0.347, P = 0.031), GLevNonU of T2 map (R2 = 0.366, P = 0.028), LngREmph of T2star map (R2 = -0.367, P = 0.028), LngREmph of R2star map (R2 = -0.356, P = 0.033) and Fraction of T1rho map (R2 = 0.36, P = 0.033), whereas no significant correlations in other measurements.Discussion
We used 3D texture analysis of MRI relaxation times, combined with clinical outcomes, to evaluate the potential of repair cartilage with allogeneic haMPCs in patients with KOA. In our study, significant differences were observed in texture RLM parameters of T1rho and T2 maps in patients, including RLNonUni, LngREmph and ShrtREmp, suggesting a possible changes of spatial distribution in cartilage composition with this treatment (Fig. 2). This result was consistent with recent studies that suggested the uniformity of both T1rho and T2 mappings was associated with the degree of cartilage degeneration5.The correlations between WOMAC pain scores and texture parameters as shown Fig. 3 demonstrated the spatial heterogeneity of relaxation time maps maybe associated with clinical scores, whereas no such correlation was found in the mean relaxation values in our previous study4. This was consistent with Joseph’s conclusion that the heterogeneous nature of cartilage tissue, compared with mean values, is more important consideration when quantifying cartilage tissue integrity6.
No significant differences of D value analysis of T2star (or R2star) were found between the three groups (Fig. 2), suggesting T2star (or R2star) mapping was less sensitive than other mapping in differentiating difference between three dose groups. Therefore, more scientific evidence is needed to definitively determine the meaning and clinical significance of T2star (or R2star) and their correlation with cartilage degeneration.
Conclusion
MRI texture analysis of cartilage may allow detection of the compositional variation of repair cartilage with treatment of allogeneic haMPCs. This has potential applications in understanding mechanism of stem cells repairing cartilage and assessing response to treatment.Acknowledgements
This work was supported by the Clinical Research Project of Health Industry of Shanghai Health Committee (Grant No. 20194Y0087 to X. Z.).
References
1. Carballido-Gamio, J., Joseph, G. B., Lynch, J. A., Link, T. M. & Majumdar, S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med 65, 1184-1194, doi:10.1002/mrm.22693 (2011).
2. MacKay, J. W. et al. MRI texture analysis of subchondral bone at the tibial plateau. Eur Radiol 26, 3034-3045, doi:10.1007/s00330-015-4142-0 (2016).
3. MacKay, J. W. et al. Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur Radiol 28, 4687-4695, doi:10.1007/s00330-018-5444-9 (2018).
4. Zhao, X. et al. Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res Ther 10, 308, doi:10.1186/s13287-019-1406-7 (2019).
5. Li, X. et al. Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med 61, 1310-1318, doi:10.1002/mrm.21877 (2009).
6. Joseph, G. B. et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls--data from the osteoarthritis initiative. Arthritis Res Ther 13, R153, doi:10.1186/ar3469 (2011).
Figures
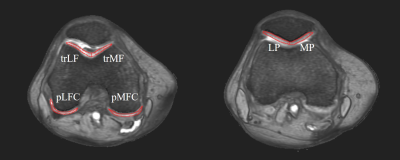
Definition of regions of interest of cartilage on transverse fat suppressed T2-weighted images. trLF: lateral side of trochlea; trMF: medial side of trochlea; LFC: lateral femoral condyle; MFC: medial femoral condyle; LP: lateral side of patella; MP: medial side of patella.

