3079
Aberrant Intrinsic Functional Connectivity of Hippo-Orbitofrontal-Insular Circuit in Drug-naive Adolescents with Major Depressive Disorder1Department of Radiology, Huaxi Magnetic Resonance Research Centre (HMRRC),Functional and Molecular Imaging Key Laboratory of Sichuan Province, Chengdu, China, 2Department of Radiology, the Third Hospital of Mianyang, Sichuan Mental Health Center, Mianyang, China
Synopsis
Through seed-based functional connectivity (SBFC) analysis, we investigated the altered intrinsic connectivity patterns of hippocampus, orbitofrontal cortex (OFC) and insula in adolescents with major depressive disorder(aMDD) comparing to typically developing control(TDC). We found distinct network alterations of those three structures at regional and subregional level. Moreover, we located the aberrant connectivity in hippo-orbitofrontal-insular circuit with family conflict and depressvie symptoms in aMDD which may underline the neural mechanism for depressvie symptoms in adolescents.
Introduction
The dysfunctions of network connectivity based on the hippocampus, orbitofrontal cortex(OFC) and insula have been associated widely with emotional and cognitive disturbances in major depressive disorder(MDD)1,2,3. However, relatively little is known about the distinct roles of these three regions at rest in MDD at the stage of adolescence. Besides, the priors also less focused on the alterations of whole brain intrinsic connectivity in the subfields of these three areas. Whereas, the distinct functions of medial and lateral OFC in reward-processing related to MDD had been reported by several studies4, and as a relatively large cortical and subcortical region, insular and hippocampal subfields represent different functions at the anterior-posterior axis had also been mentioned before5,6. Therefore, in the current study, we used the seed-based functional connectivity analysis to delineate the precise contributions of hippocampus, orbitofrontal cortex and insula at both regional and sub-regional level to explore the underlying neurobiological mechanism of adolescent depression.Materials & Methods
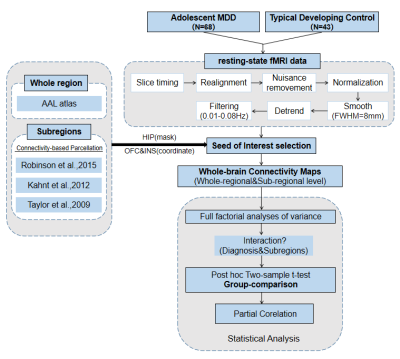
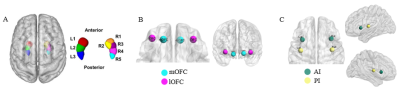
We recruited 68 first-episode drug-naive adolescent patients with MDD and 43 age- and gender- matched typically developing control(TDC). All the participants underwent 3.0-Telsa Siemens magnetic resonance imaging(MRI) system equipped with 20-channel phased-array head coil to acquire resting-state functional MRI(rs-fMRI) and high-resolution structural MRI data. The rs-fMRI data preprocessing was performed following the procedure showed in Figure 1. Furthermore, we selected the whole hippocampus, OFC and insula as the region of interests (ROIs) using the AAL atlas. Specifically, we defined 2 subregional seeds of OFC7 and insula5 in each hemisphere in accordance with connectivity-based parcellation of these three structures which had reported before (Figure.1). Both OFC and insula seeds were identified as spheries with radius=6mm, and located in MNI space as the following coordinates: left/right medial OFC (mOFC.L/R, [-17,42,-13], [11,41,-15]) and left/right lateral OFC(lOFC.L/R, [-36,44,-10], [33,42,-9]) as well as left/right anterior insula (AI.L/R, [-34,14,2], [36,16,2]) and left/right posterior insula (PI.L/R, [-38,-12,7], [38,-10,7]) (Figure 2). The hippocampus was defined as 3 subregions in the left hemisphere and 5 subregions in the right hemisphere according to Robinson6 (Figure 2). Whole-brain resting-state functional connectivity(RSFC) maps of these three regions at both regional and sub-regional level were generated for all subjects. Then we apply full factorial analyses of variance to test the interaction between diagnosis and subdivision To characterize different functional connectivity patterns of hippocampal, orbitofrontal and insular subdivisions between adolescent MDD patients and TDC, post hoc two-sample t-test voxel-based comparisons of functional connectivity maps of the subdivisions of these three regions were performed subsequently (Figure 1). The significance threshold was set to P<0.005 at the voxel level, and few correction was set to P<0.05 at the cluster level. Eventually, we used partial correlations (two-tailed) to explore the association between clinical variables and regions showing group differences in adolescent MDD group, after controlling for age and gender.Results
The socio-demographic and clinical characteristics of the participants were provided in Table 1. There was no significant interaction between diagnosis and subregions through full factorial analyses of variance. Compared to TDC, aMDD patients showed the weaker connectivity between left hippocampus with bilateral medial OFC and right inferior temporal gyrus (ITG) while the stronger connectivity between right hippocampus R3 with right posterior insula. In addition, we found hypoconnectivity of the left medial OFC with a large cluster including left insula and inferior temporal gyrus (IFG) extending to lentiform nucleus as well as the hyperconnectivity in the right anterior insula and right hippocampus of adolescent depressive patients. However, there were no significantly difference for bilateral OFC FC and insula FC between two groups (Figure 3). Via partial correlation analysis, we discovered that weaker connectivity between left hippocampus and bilateral medial OFC correlated with increased family conflict in adolescent depressive patients (P<0.05, r=-0.278). And the connectivity of left medial OFC and left insula and ITG correlated negatively with total score (P<0.05, r=-0.243) and insomnia factor (P<0.05, r=-0.250) of HAMA (Figure 3).Discussion & Conclusion
Using a seed-based approach, we identified alterations of intrinsic connectivity in the hippocampus, OFC and insula at regional and subregional level in adolescent MDD compared to TDC. The specific impairments mainly restricted in the hippo-orbitofrontal-insular circuit (Figure 4). This circuit altered connectivity may be associated with several aspects of affective and cognitive disturbances in adolescent depression including abnormal memory-related stress regulation, failed reward processing and interoceptive integration. These findings provided evidence for the pathophysiological role of the hippo-orbitofrontal-insular circuit in adolescents with MDD and may provide target for developing new treatment strategy for adolescents with MDD.Acknowledgements
This study is supported by grants from 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21041) and Clinical and Translational Research Fund of Chinese Academy of Medical Sciences (2021-I2M-C&T-B-097).References
1. Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004 Nov;29(6):417-26.
2. Pizzagalli DA, Roberts AC. Prefrontal cortex and depression. Neuropsycho pharmacology. 2021 Aug 2.
3. Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012 Jun 29;520(2):204-9.
4. Cheng W, Rolls ET, Qiu J, Liu W, Tang Y, Huang CC, Wang X, Zhang J, Lin W, Zheng L, Pu J, Tsai SJ, Yang AC, Lin CP, Wang F, Xie P, Feng J. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain. 2016 Dec;139(Pt 12):3296-3309.
5. Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009 Sep;30(9): 2731-45.
6. Robinson JL, Barron DS, Kirby LA, Bottenhorn KL, Hill AC, Murphy JE, Katz JS, Salibi N, Eickhoff SB, Fox PT. Neurofunctional topography of the human hippocampus. Hum Brain Mapp. 2015 Dec;36(12): 5018-37.
7. Kahnt, T., et al. (2012). "Connectivity-based parcellation of the human orbitofrontal cortex." J Neurosci 32(18): 6240-6250.
Figures
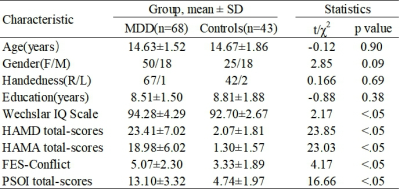
Table 1 Socio-demographic information and clinical characteristics of adolescent major depressive disorder (aMDD) and typical developing controls (TDC).
Abbreviations: SD: standard deviation; HAMD: Hamilton Depression Rating Scale; HAMA: Hamilton Anxiety Rating Scale; FES: Family Environment Scale; PSQI: Pittsburg Sleep Quality Index.
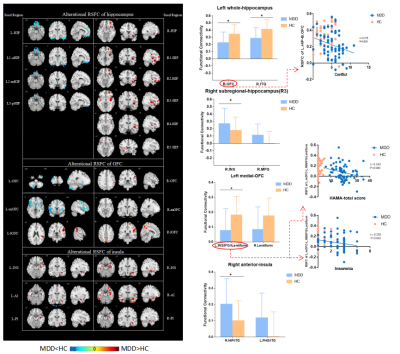
Note. * significant differences through the FWE correction.
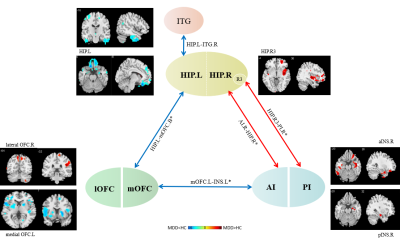
Note: full-line: PFWE-correction<0.05; vacant-line: Puncorrected<0.05.
blue arrow: hypoconnectivity; red arrow: hyperconnectivity.