3072
The disrupted subcortical functional gradient in schizophrenia and the normalization effects of antipsychotic treatment1Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, China., Chengdu, China, 2College of Electronic Engineering, Chengdu University of Information Technology, Chengdu, P.R. China., Chengdu, China
Synopsis
To characterize the connectome gradient changes of subcortical system in drug-naive first-episode schizophrenia and the longitudinal treatment effect, we calculated the functional gradient and conducted the group comparisons. The principal gradient of subcortex demonstrated the fundamental functional segregation differences between patients at baseline and healthy controls, and the longitudinal analyses indicated that the treatment would gradually normalize the altered gradients with significant improvement of symptoms. This study provided the new perspective on the abnormal subcortical hierarchy organization in schizophrenia and its longitudinal gradient changes could be sensitive to reflect the antipsychotic treatment effect.
Background
Schizophrenia is a chronic and severe psychotic disorder that affects approximately 1% of the population, and has a profound impact on individuals who have been affected1. Growing evidence indicates that subcortex is pivotal in the development of schizophrenia as the dopamine regulation center2, and changes in subcortical regions were also sensitive indicators of pharmacological treatments that are dopamine D2-receptor blockers3. Neuroimaging studies focusing on regional brain function or functional connectivity have showed subcortical alterations and the association with antipsychotic drug efficacy4,5. However, how the subcortex embeds within the macroscale cortical system, and the structural constraints and functional emergences of spatial arrangements, remain unclear. The new concept of gradients focused on area spatial relationships, which revealed that macroscale gradients integrate systematic information into more abstract representations6. Exploring the subcortex in the broader cortical landscape as well as investigating its feature as treatment target in schizophrenia is promising for its extensive connections with the entire cerebral cortex7. Therefore, we aimed to characterize the connectome gradient changes of subcortical system that provide the topological representations of subcortical macroscale hierarchy after short- and long-term treatment.Methods
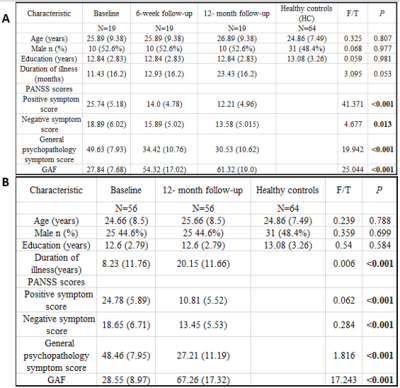
We recruited nineteen drug-naive first-episode schizophrenia (FES), and their resting-state functional MRI (rs-fMRI) and T1-weighted images (T1WI) were collected at baseline (FES0w), 6 week (FES6w) and 12-month (FES12m) after administrating routine antipsychotic treatment. In addition, the sample of baseline (FES0w2) and 12 month (FES12m2) was expanded to fifty-six to further testified the long-term treatment effects. Sixty-four healthy controls (HC) were included and scanned with the same protocol as patients. All participants’ demographic information and clinical variables were showed in Figure 1. The functional data were preprocessed, including removal of the first five frames, slice timing, motion correction using rigid body translation and rotation, and boundary-based registration to T1WI. Furthermore, the functional connectome gradients were calculated by BrainSpace toolbox8, and then compared between patients and HC to characterize the illness effects. Short- (6 week) and long-term (12 month) treatment effects were examined by calculating the longitudinal changes in functional connectome gradients before and after treatment. Correlation analysis was conducted between the longitudinal functional gradient alterations and the psychopathological ratings, including Positive and Negative Syndrome Scale (PANSS) and Global Assessment of Functioning (GAF) scores.Results
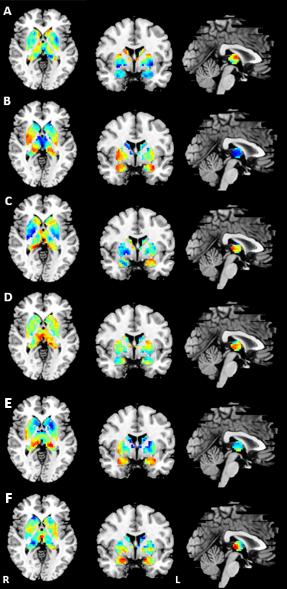
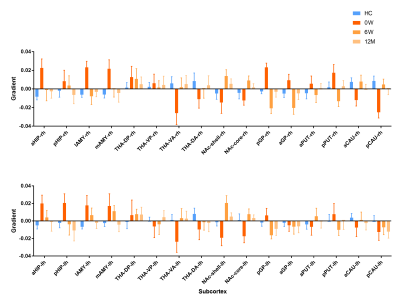
The principal gradient of subcortex in FES0w significantly differed from HC. Compare to HC, there was a significant increase in FES0w mainly in bilateral hippocampus, amygdala, globus pallidus, putamen and decrease mainly in bilateral thalamus, nucleus accumbens and caudate (all p < 0.05, Bonferroni corrected). When compared the functional gradients of FES0w with FES6w and FES12m respectively, the abnormality was gradually inclined to HC after 6-week and 12-month antipsychotic treatment, especially in bilateral hippocampus (all p < 0.05, Bonferroni corrected). The difference between FES0w2 and FES12m2 further enhanced the same observation of functional gradient improves after long-term treatment, primary in bilateral hippocampus (all p < 0.05, Bonferroni corrected). The functional gradient results of group comparison showed in Figure 2 and 3. Then, the increased gradients in FES6w and FES12m were positively while the decreased gradient values were negatively correlated with symptom improvement, that is to say, with less positive symptoms and less general psychopathology symptoms as rated with PANSS scores and higher GAF scores.Discussion
The current study characterized the topographic representations of the subcortex based on subcortical-cortical functional connectomes. Different samples confirmed the principal gradients enhanced homogeneity in bilateral limbic system, thalamus as well as right striatum, which revealed the fundamental functional segregation differences between FES0w and HC. These abnormalities further emphasized core impairment function of schizophrenia, such as impaired memory, emotion and cognition9,10. Furthermore, we found that longitudinal analyses in functional gradient of subcortex indicated the short- and long-term treatment would gradually normalize the altered gradients with significant improvement of symptoms. Specifically, the putamen, caudate nuclei and accumbens are parts of the striatum with rich dopamine receptors, which are recognized as antipsychotic treatment targets for schizophrenia11. Consistent with this finding, our study suggested that the striatal homogeneous function reinforced the power on dopamine circuit at baseline, but nearly returned to normal level after antipsychotic treatment in schizophrenia, which demonstrated that drugs may improve functional coordination of striatum on the subcortical macroscale hierarchy.Conclusion
Our findings provided a novel insight into the functional system hierarchy alterations of subcortical organization, which were sensitive to illness and treatment effects. This might extend our understanding of the functional connectome hierarchy of subcortex in schizophrenia, and this measure makes a promising indicator of treatment response.Acknowledgements
NoneReferences
1. Owen MJ, Sawa A, Mortensen PB. Schizophrenia. Lancet (London, England). 2016;388:86-97.
2. Chestnykh DA, Amato D, Kornhuber J, Müller CP. Pharmacotherapy of Schizophrenia: Mechanisms of Antipsychotic Accumulation, Therapeutic Action and Failure. Behavioural brain research. 2021;403:113144.
3. Goff DC. The Pharmacologic Treatment of Schizophrenia-2021. Jama. 2021;325:175-176.
4. Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, et al. Short-Term Effects of Antipsychotic Treatment on Cerebral Function in Drug-Naive First-Episode Schizophrenia Revealed by "Resting State" Functional Magnetic Resonance Imaging. Arch Gen Psychiatry. 2010;67:783-792.
5. Li F, Lui S, Yao L, Hu J, Lv P, Huang X, et al. Longitudinal Changes in Resting-State Cerebral Activity in Patients with First-Episode Schizophrenia: A 1-Year Follow-up Functional Mr Imaging Study. Radiology. 2016;279:867-875.
6. Huntenburg JM, Bazin PL, Margulies DS. Large-Scale Gradients in Human Cortical Organization. Trends in cognitive sciences. 2018;22:21-31.
7. Tian Y, Margulies DS, Breakspear M, Zalesky A. Topographic Organization of the Human Subcortex Unveiled with Functional Connectivity Gradients. Nature neuroscience. 2020;23:1421-1432.
8. Vos de Wael R, Benkarim O, Paquola C, Lariviere S, Royer J, Tavakol S, et al. Brainspace: A Toolbox for the Analysis of Macroscale Gradients in Neuroimaging and Connectomics Datasets. Communications biology. 2020;3:103.
9. Tregellas JR, Smucny J, Harris JG, Olincy A, Maharajh K, Kronberg E, et al. Intrinsic Hippocampal Activity as a Biomarker for Cognition and Symptoms in Schizophrenia. Am J Psychiatry. 2014;171:549-556.
10. Ren W, Lui S, Deng W, Li F, Li M, Huang X, et al. Anatomical and Functional Brain Abnormalities in Drug-Naive First-Episode Schizophrenia. Am J Psychiatry. 2013;170:1308-1316.
11. Li M, Chen Z, Deng W, He Z, Wang Q, Jiang L, et al. Volume Increases in Putamen Associated with Positive Symptom Reduction in Previously Drug-Naive Schizophrenia after 6 Weeks Antipsychotic Treatment. Psychol Med. 2012;42:1475-1483.
Figures