3071
Linked brain connectivity patterns with psychopathological and cognitive phenotypes in drug-naïve first-episode schizophrenia1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan University, Chengdu, China
Synopsis
Schizophrenia is characterized by abnormal functional integration between distinct brain regions but whether a common deficit in functional connectivity in relation to both clinical symptoms and cognitive impairments would present in drug-naïve first-episode patients remains elusive. A connectome-wise analysis on resting-state functional MRI in never-treated patients with first-episode schizophrenia. Using the principal component analysis, we found a trans-symptomatic pattern of functional connectivity associated with both psychopathological and cognitive manifestations in never-treated first-episode schizophrenia characterized as the dysconnections involving frontal and visual cortices, suggesting a core deficit of brain functional connectivity that might underpin the psychopathology of schizophrenia.
Introduction
Schizophrenia is widely considered to be a disorder of dysconnectivity characterized by abnormal functional integration between distinct brain regions 1. The identification of brain connection abnormalities in patients and their associations with the psychopathological symptoms or cognitive deficits has been of sustained interest 2-5. Studies on functional brain image suggested that although different dimensions of clinical manifestations have specific relevant deficits in brain network, they might share some common impairment patterns 6-9. Moreover, different dimensions of symptoms should be intercorrelated, rather than independent to each other 10. However, it remains unclear whether a common deficit in functional connectivity (FC) in relation to both clinical symptoms and cognitive impairments would present in first-episode patients who have never received any medication.Methods
A total of 75 patients with first-episode schizophrenia underwent resting-state brain imaging scans including high-resolution 3D T1-weighted and T2-weighted structure images and Resting-state functional MRI. After preprocessing, intrinsic FC matrices were constructed. All the patients were accessed by a series of clinical rating scales including the Positive and Negative Syndrome Scale (PANSS), Global Assessment of Functioning (GAF), Hamilton Depression Scale (HAMD), Hamilton Anxiety Scale (HAMA) and the Brief Assessment of Cognition in Schizophrenia (BACS) scale. Partial correlation analyses were conducted between the intrinsic FC matrices and clinical scale ratings of different symptom dimensions. A principal component analysis (PCA) procedure was performed on the derived correlation coefficient matrices to identify the potential core correlation pattern linking functional connectome and both psychopathological and cognitive manifestations.Results
Using the PCA approach, the first principal component (PC1) explained 37% of the total variance in the correlation matrices across all the 7 clinical features. In total, 14 connections in PC1 survived from permutation testing that were significantly correlated with clinical ratings (p<0.05, Bonferroni corrected), including 3 connections with positive scores and 11 connections with negative scores (Figure 2). The connections with positive PC scores were negatively associated with BACS and GAF while positively with PANSS, HAMA and HAMD ratings, including those between right gyrus rectus (R.REC) and left anterior cingulate cortex (L.ACC), between right orbital part of middle frontal gyrus (R.OrbMFG) and right triangular part of inferior frontal gyrus (R.TriIFG), and between right supplementary motor area (R.SMA) and right middle temporal gyrus (R.MTG). The connections with negative PC scores were positively associated with BACS and GAF while negatively with PANSS, HAMA and HAMD ratings. These connections mainly involved visual cortices (including right cuneus [R.CUN], right middle and bilateral superior occipital gyrus [R.MOG, L.SOG and R.SOG], and right inferior temporal gyrus [R.ITG]) and anterior and middle cingulate cortices (ACC and MCC).Discussion
Stronger connectivity in connections involving multiple regions in frontal cortex indicated severe cognitive and functional impairments with more psychopathological and emotional symptoms. Connections involving visual associated occipital areas were associated positively with better cognition/function and less emotional and psychopathological symptoms. While further replication of our findings is warranted in studies with larger samples, our study provided initial evidence for a common network change underlying various domains of clinical phenotypes in patients. The heterogeneity of schizophrenia manifests great severity in patients’ behavioral deficits that were mainly characterized by either positive or negative symptoms, or both, together with different extents of cognitive deficits. To disentangle the neural substrates underlying the clinical manifestations of schizophrenia has been a great challenge but with limited progress. In such efforts, we successfully extracted the general composite underlying different symptoms and cognition, and identified a linked brain connectivity patterns which could explain the covariance of these clinical phenotypes, without confounding effects of antipsychotic medication. The frontal cortex is a crucial cortical region that plays essential roles in the cognitive process, regulation of emotion, motivation, and sociability 11,12. Visual cortex is believed to have multisensory function beyond visual processing, which directly impacts behavior and perception 13-16. Our findings of dysfunction of frontal and visual cortex related connections might represent the common abnormalities in the functional connections that underpin the primary behavioral abnormalities in early schizophrenia.Conclusion
In conclusion, we found a trans-symptomatic pattern of FC associated with both psychopathological and cognitive manifestations in never-treated first-episode schizophrenia characterized as the dysconnections related to frontal and visual cortex, which may represent a core deficit of brain FC in schizophrenia patients. Detecting this general pattern of brain changes might help achieve early diagnosis of the disorder, and develop targeted therapeutic interference accordingly to prevent further disease progression.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant Nos. 8212018014, 82071908, 81761128023 and 82101998), Sichuan Science and Technology Program (Grant Nos. 2021JDTD0002 and 2020YJ0018), the Science and Technology Project of the Health Planning Committee of Sichuan (Grant No. 20PJ010), Post-Doctor Research Project, West China Hospital, Sichuan University (Grant No. 2020HXBH005), the Fundamental Research Funds for the Central Universities (Grant No. 2020SCU12053), Postdoctoral Interdisciplinary Research Project of Sichuan University (Grant No. 0040204153248) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Project Nos. ZYYC08001 and ZYJC18020). Dr. Lui acknowledges the support from Humboldt Foundation Friedrich Wilhelm Bessel Research Award and Chang Jiang Scholars (Program No. T2019069).References
1. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neurosci Biobehav Rev Apr 2011;35(5):1110-1124.
2. Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res Oct 2016;176(2-3):83-94.
3. Li S, Hu N, Zhang W, et al. Dysconnectivity of Multiple Brain Networks in Schizophrenia: A Meta-Analysis of Resting-State Functional Connectivity. Front Psychiatry 2019;10:482.
4. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr Bull Jan 13 2018;44(1):168-181.
5. Chen J, Wensing T, Hoffstaedter F, et al. Neurobiological substrates of the positive formal thought disorder in schizophrenia revealed by seed connectome-based predictive modeling. Neuroimage Clin 2021;30:102666.
6. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev Feb 2016;61:108-120.
7. Yamashita M, Shimokawa T, Takahashi S, Yamada S, Terada M, Ukai S, Tanemura R. Cognitive functions relating to aberrant interactions between task-positive and task-negative networks: Resting fMRI study of patients with schizophrenia. Appl Neuropsychol Adult Dec 5 2020:1-9.
8. Hare SM, Ford JM, Mathalon DH, et al. Salience-Default Mode Functional Network Connectivity Linked to Positive and Negative Symptoms of Schizophrenia. Schizophr Bull Jun 18 2019;45(4):892-901.
9. Wang D, Li M, Wang M, et al. Individual-specific functional connectivity markers track dimensional and categorical features of psychotic illness. Mol Psychiatry Sep 2020;25(9):2119-2129.
10. Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res Sep 1991;5(2):123-134.
11. Xu P, Chen A, Li Y, Xing X, Lu H. Medial prefrontal cortex in neurological diseases. Physiol Genomics Sep 1 2019;51(9):432-442.
12. Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain Feb 1995;118 ( Pt 1):279-306.
13. Murray MM, Thelen A, Thut G, Romei V, Martuzzi R, Matusz PJ. The multisensory function of the human primary visual cortex. Neuropsychologia Mar 2016;83:161-169.
14. Keane BP, Cruz LN, Paterno D, Silverstein SM. Self-Reported Visual Perceptual Abnormalities Are Strongly Associated with Core Clinical Features in Psychotic Disorders. Front Psychiatry 2018;9:69.
15. Turkozer HB, Hasoglu T, Chen Y, et al. Integrated assessment of visual perception abnormalities in psychotic disorders and relationship with clinical characteristics. Psychol Med Jul 2019;49(10):1740-1748.
16. van de Ven V, Rotarska Jagiela A, Oertel-Knochel V, Linden DEJ. Reduced intrinsic visual cortical connectivity is associated with impaired perceptual closure in schizophrenia. Neuroimage Clin 2017;15:45-52.
Figures
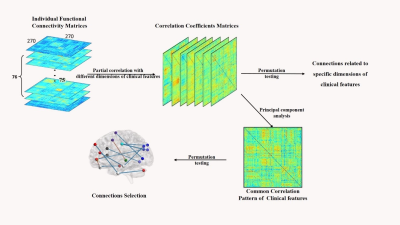
Figure 1. Data processing pipeline for detecting relationship between FCs and clinical phenotypes.
PCA, Principal Component Analysis.
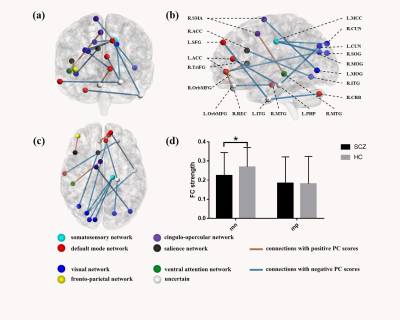
Figure 2. Connections related to clinical features in the first principal component.
(a) coronal view, (b) sagittal view, and (c) axil view of the connections significant in PC1;
The balls represented nodes of the brain area and the different colors indicated the modules according to the atlas. Sticks represented connections between two brain areas.
(d) between-group comparison of the mean FC strength of connections with positive or negative PC scores.
mn/mp, mean FC of connections with negative/positive PC scores; *, p value of ANCOVA was 0.014, Bonferroni corrected.