3070
Decreased the stability of brain hubs and the heterogeneity of whole brain dynamics in early onset schizophrenia1Department of NMR & MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Department of psychiatry, All India Institute of Medical Sciences, New Delhi, India
Synopsis
The whole-brain effective connectivity (EC) was estimated to interpret the brain dynamics, time-dependent communicability and propagation of neuronal activity within the brain state in patients with early onset schizophrenia. We observed decreased effective connectivity (p<0.05) and global integration of local endogenous events in early onset schizophrenia (EOS) as compared to controls. EOS suffer decreased propagation of neuronal activity and responsiveness to events.
Introduction
Schizophrenia represents a chronic disorder affecting nearly 1% of the world’s population. Schizophrenia appearing in less than 18 years of age is referred early onset schizophrenia (EOS). EOS have more severe premorbid neurodevelopmental abnormalities, worse long-term outcome, more cytogenetic anomalies, and potentially greater loading of family histories for schizophrenia and associated spectrum disorders than their adult counterparts1,2. Previous network-oriented analysis study has been reported reduced functional connectivity in sensory regions, default mode, frontoparietal and cingulate-opercular network3,4 in EOS. Altered dALFF in the right middle temporal gyrus (MTG)3 has also been reported. We used whole-brain effective connectivity (EC) model (comprising whole brain integration, segregation, metastability and intrinsic ignition) to interpret the brain dynamics in 16 patients of EOS to understand time-dependent communicability and propagation of neuronal activity within the brain state.Materials and Methods
Sixteen EOS patients (mean age 17.68±2.15 years; 6F/10M) who met ICD-10 (code F20) criteria with positive (hallucinations, delusions, altered thoughts) and negative (social withdrawal, loss of speech) symptoms were recruited from the psychiatric clinics. Ten healthy subjects (age=20.9±0.99, 4F/6M) with no history of neurological and psychiatric disorder were recruited from local community.Data was acquired on a 3T MRI (Ingenia, Philips) using 32-channel head coil after obtaining informed consent. Functional MRI volumes were acquired using echo-planar T2* -weighted sequence with 35 slices of 4 mm thickness, 210 dynamics, TE/TR:30/2000 ms, FOV = 250 mm, flip angle =90. Tl weighted image consisting of 350 slices was also acquired.
Resting data was analyzed with MELODIC and Structural Connectivity Matrix computed with FMRIB’s Diffusion Toolbox (www.fmrib.ox.ac.uk/fsl). Functional resting state atlas was used for parcellation to obtain the BOLD time series of the 214 cortical and subcortical brain areas in each individual's native EPI space, their time series extracted5 and a group structural connectivity (SC) mask was obtained by averaging the all-control subjects' SC matrix and applying a threshold of 80% to maintain the top 20% of strongest connections to binarize the SC6 for the computation of EC.
Result
Altered causal interactions of whole-brain effective connectivity was observed in EOS patients. We observed decreased whole brain integration (p=.021), increased whole brain segregation (p=.016) (Figure 1a, b), decreased whole brain metastability (p=.027) (Figure 1c) and low whole brain intrinsic ignition (p=.027) (Figure 1d) in EOS as compared to controls.In-silico perturbation applied to individual ROIs, spread along the network and the effect of regional perturbations on the rest of the network were estimated. The finding showed an altered global integration of local endogenous events in early onset schizophrenia as compared to controls. Global brain responses (Figure 2b) undergo a transient peak (immediately after the initial perturbations) and then decay as the effects of the stimuli dilute with time and the system relaxes back to its stationary state. This relaxation is also observed by the homogenization of the response matrices during the longer latencies (Figure 3a). The global response curves exhibited different pattern in controls and EOS.
Discussion
Neural propagation of endogenous and exogenous perturbations in the brain was studied using model-free and model-based analysis methods, to elucidate the mechanisms behind early onset schizophrenia. Reduced functional connectivity in sensory regions, default mode network (DMN), frontoparietal network and the cingulate-opercular network has been reported4,7. Altered dALFF was observed in bilateral precuneus, right middle temporal gyrus (MTG)3 in EOS. But the understanding of the mechanisms behind EOS is still missing.We studied the propagation of endogenous and in-silico exogenous perturbations in patients with EOS, based upon directed and causal interactions estimated from resting-state fMRI. This provides time-dependent communicability and propagation of neuronal activity within the brain state. The method employed to investigate the directional causal interactions between brain regions, thus elucidating alterations in the broadcasting and the integrating capacities of individual areas or pathways, between normal control and EOS patients. These results show that patients with EOS have a decreased capacity for neural propagation and responsiveness to brain states.
Conclusion
The cerebral capacity of propagation of neuronal activity and integration of global and local (naturally occurring) events is affected in EOS, with respect to that in healthy controls.Acknowledgements
We acknowledge that this research was supported by the National DBT-RA Program Biotechnology and Life Sciences, Ministry science and technology, Government of India.
References
1. Keshavan, M.S et al. Journal of psychiatric research. 1994;28 (3): 239-265.
2. Cannon, T.D. et al. Schizophrenia bulletin. 2003; 29 (4): 653-669.
3. Li, Q. et al. Frontiers in neuroscience. 2020; 14: 901
4. Wang, X. et al. Schizophrenia research. 2019; 208: 308-316
5. Finn, E.S. et al. Nature neuroscience. 2015; 18(11): 1664-1671.
6. Gilson, M. et al. NeuroImage. 2019; 201: 116007.
7. Jiang, S. et al. Journal of Central South University. Medical sciences 2010; 35(9): 947-951
Figures
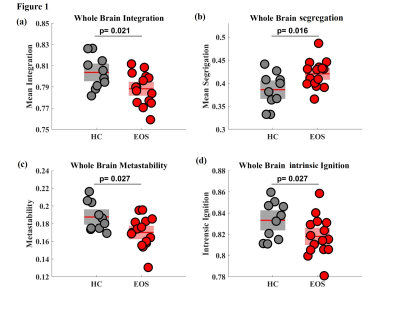
Figure 1. Dynamics of resting state fluctuations in the brain of EOS patients with respect to controls (a) Whole brain integration, (b) Whole brain segregation, (c) Whole brain metastability and (d) Whole brain intrinsic ignition. Each dot represents a participant and the boxes represent the distribution. Boxplots represent mean of the measures with a 95% confidence interval (red) and SD (light). Intergroup differences were assessed using t-test (p < 0.05 FDR corrected). HC=Healthy control; EOS=Early onset Schizophrenia
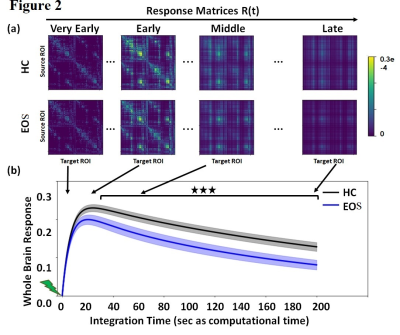
Figure 2. In-silico perturbations. (a) Temporal evolution of the response matrices for HC and EOS patients at different times (early = 2 sec, middle = 20 sec, late 60 sec and very late = 200 sec). Color-bar represents the relative strength of the response between brain regions. (b) Whole brain response curves for the control and EOS reflecting the sum of all pair-wise responses at each time point post-stimulus. (Bonferroni corrected for 100 tests/time points, p-value=0.05). HC=Healthy control; EOS=Early onset Schizophrenia