3066
Fixel-Based Analysis Identifies White Matter Tract Degeneration in Parkinson’s Disease with Mild Cognitive Impairment
Chih-Chien Tsai1,2, Ting-Wei Liao3, Yih-Ru Wu3,4, Yi-Ming Wu5, and Jiun-Jie Wang2,6,7
1Department of Medical imaging and Radiological Science, Chang Gung University, Taoyuan City, Taiwan, 2Healthy Aging Research Center, Chang Gung University, Taoyuan City, Taiwan, 3Department of Neurology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 4College of Medicine, Chang Gung University, Taoyuan City, Taiwan, 5Department of f Medical Imaging and Intervention, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 6Department of Medical imaging and Radiological Science, Chang-Gung University, Taoyuan City, Taiwan, 7Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University/Chang Gung Memorial Hospital, Taoyuan City, Taiwan
1Department of Medical imaging and Radiological Science, Chang Gung University, Taoyuan City, Taiwan, 2Healthy Aging Research Center, Chang Gung University, Taoyuan City, Taiwan, 3Department of Neurology, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 4College of Medicine, Chang Gung University, Taoyuan City, Taiwan, 5Department of f Medical Imaging and Intervention, Chang Gung Memorial Hospital, Taoyuan City, Taiwan, 6Department of Medical imaging and Radiological Science, Chang-Gung University, Taoyuan City, Taiwan, 7Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University/Chang Gung Memorial Hospital, Taoyuan City, Taiwan
Synopsis
Cognitive decline is a common non-motor symptom in Parkinson's disease (PD) patients, described as mild cognitive impairment (PD-MCI). PD-MCI is identified as a risk for PD with Dementia. Previous studies indicated that PD-MCI patients showed gray matter volume decrease and white matter tract degeneration. We investigate the utility of fixel based analysis as a biomarker for disease progression. The results show a significant degeneration of white matter in PD-MCI patients. Our findings indicated the white matter tract degeneration in PD-MCI patients and shed light on the value of the fiber-specific method to the clinical diagnosis of PD-MCI.
INTRODUCTION
PD-MCI is estimated to be as high as 20% to 42% at the time of PD diagnosis [1]. PD-MCI is identified as a risk for PDD, and half of the PD patients have progressed to PDD after ten years [2; 3; 4]. Cognitive impairment in PD has a more significant effect than motor symptoms on the patient's quality of life, intensifies caregiver burden, and is associated with increased mortality. Therefore, it is important to identify these patients early. Diffusion tensor imaging (DTI) showed that white matter damages in the corpus callosum, corona radiata, and the inferior and superior longitudinal fasciculi can be consistently noticed in patients with PDD and PD-MCI [5]. Besides, DTI studies also help in the early recognition of cognitive decline. Although PDD patients had reduced GM volume and fractional anisotropy (FA), PD-MCI patients only showed FA abnormalities in the main WM tracts, suggesting that tract damage may precede GM atrophy [6]. WM impairment in PD might be a sensitive sign preceding the neuronal loss in associated GM regions [7]. However, DTI can be limited by its lack of capability to model complex and crossing-fiber populations, shown to be present in up to 90% of white matter voxels [8]. To overcome these limitations, fixel-based analysis (FBA) is a recently developed technique that enables fiber tract-specific statistical analysis [9]. Three quantitative metrics can be derived and provide a more comprehensive insight into the white matter alterations, either micro- or macro-structurally [9]. FBA might potentially improved anatomical specificity and sensitivity to the white matter abnormalities than the traditional DTI method [10] . We hypothesized that altered white matter integrity in a specific area would be associated with cognitive decline in patients with PD-MCI. Moreover, deficits in the different cognitive domains might be related to other areas of fiber tract alterations.METHODS
The study protocol complied with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board. All participants gave their written informed consent. A total of 75 patients with PD completed the evaluation and entered the final analysis. Forty-two patients were diagnosed as PD-MCI according to the level I clinical criteria from International Parkinson and Movement Disorders Society . MRI was performed with a 3T scanner with a 15‐channel receive‐transmit head coil (Ingenia; Philips, Amsterdam, Netherlands). Diffusion-weighted images were acquired using a spin-echo echo-planar-imaging sequence with repetition time/echo time/slice thickness = 4000 ms/77.2 ms/ mm, matrix size = 128 × 128, and field of view = 256 × 256 mm2, 64 slices which covered the whole brain down to the cerebellum. Diffusion-weighted gradients were applied along 64 non-collinear directions using b-values of 0 and 1000 s/mm2. All patients also underwent clinical examinations and neuropsychological evaluations, including the Mini-Mental State Examination, Montreal Cognitive Assessment, and at least two tests among each of the five cognitive domains. Fixel-based analysis, performed by MRtrix3 [9], was used to investigate fiber tract alterations and compared between PD-MCI and PD-NC. The fixel-based metric included fiber density (FD), fiber bundle cross-section (FC), and combined measure of FD and FC, FDC was calculated. Statistical analyses was performed with the fixelcfestat in MRtrix3. A FWE-corrected p < 0.05 was considered statistically significant [11]. Correlations with each cognitive test were also performed.RESULTS
We found that patients with PD-MCI exhibited more decreased fiber bundle cross-section (FC) values within bilateral superior corona radiata, left superior longitudinal fasciculus, and cingulum (Figure 1). In patients with PD-MCI, the California Verbal Learning Test performance had a positive correlation mostly on fiber density (FD) values involving splenium of the corpus callosum, left retrolenticular part of the internal capsule, right anterior corona radiata, sagittal stratum, and fornix. The scores of Trail Making Test -part B had a negative correlation mostly on FD and fiber density and bundle cross-sectional area (FDC) values involving corpus callosum and posterior corona radiata. In patients with PD-NC, the performance of the Boston Naming Test had a positive correlation in FC and FDC value involving genu of corpus callosum, bilateral anterior and posterior limb of the internal capsule, bilateral anterior corona radiata (Figure 2).DISCUSSION
In this study, we used FBA to compare fiber-specific changes in patients with PD-MCI with patients with PD-NC. We found that patients with PD-MCI exhibited significant white matter degeneration compared with patients with PD-NC. Both macro-and microstructural alteration were observed within body of corpus callosum and superior corona radiata. Impairment in corpus callosum was correlated with not only motor function but also multiple cognitive tests in regression analysis. Cingulum, superior longitudinal fasciculi and thalamocortical circuit had predominantly FC changes, and their impairment was associated with global cognitive decline in patients with PD. Furthermore, we found macrostructural alteration in cerebellar circuits was associated with poor motor function in patients with PD-MCI and picture naming ability in patients with PD-NC.CONCLUSION
Patients with PD-MCI have significant white matters alteration compared with patients with PD-NC. We demonstrate these changes on various bundle tracts by FBA, which may be a promising tool to detect early cognitive decline in PD. In addition, these findings help us better understand specific white matter changes that were functionally implicated in cognitive decline in patients with PD.Acknowledgements
The presents work was supported by the Imaging Core Laboratory of the Institute for Radiological Research and the Center for Advanced Molecular Imaging and Translation. The authors thank the Neuroscience Research Center (Chang Gung Memorial Hospital) and the Healthy Aging Research Center (Chang Gung University) for their invaluable support.References
[1] R.S. Weil, A.A. Costantini, and A.E. Schrag, Mild Cognitive Impairment in Parkinson's Disease-What Is It? Journa 2018; 18: 17. [2] M. Auyeung, T.H. Tsoi, V. Mok, C.M. Cheung, C.N. Lee, R. Li, et al., Ten year survival and outcomes in a prospective cohort of new onset Chinese Parkinson's disease patients. Journa 2012; 83: 607-11. [3] F. Perez, C. Helmer, A. Foubert-Samier, S. Auriacombe, J.F. Dartigues, and F. Tison, Risk of dementia in an elderly population of Parkinson's disease patients: a 15-year population-based study. Journa 2012; 8: 463-9. [4] C.H. Williams-Gray, S.L. Mason, J.R. Evans, T. Foltynie, C. Brayne, T.W. Robbins, et al., The CamPaIGN study of Parkinson's disease: 10-year outlook in an incident population-based cohort. Journa 2013; 84: 1258-64. [5] M. Delgado-Alvarado, B. Gago, I. Navalpotro-Gomez, H. Jimenez-Urbieta, and M.C. Rodriguez-Oroz, Biomarkers for dementia and mild cognitive impairment in Parkinson's disease. Journa 2016; 31: 861-81. [6] T. Hattori, S. Orimo, S. Aoki, K. Ito, O. Abe, A. Amano, et al., Cognitive status correlates with white matter alteration in Parkinson's disease. Journa 2012; 33: 727-39. [7] I. Rektor, A. Svatkova, L. Vojtisek, I. Zikmundova, J. Vanicek, A. Kiraly, et al., White matter alterations in Parkinson's disease with normal cognition precede grey matter atrophy. Journa 2018; 13: e0187939. [8] B. Jeurissen, A. Leemans, J.D. Tournier, D.K. Jones, and J. Sijbers, Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Journa 2013; 34: 2747-66. [9] D.A. Raffelt, J.D. Tournier, R.E. Smith, D.N. Vaughan, G. Jackson, G.R. Ridgway, et al., Investigating white matter fibre density and morphology using fixel-based analysis. Journa 2017; 144: 58-73. [10] L. Storelli, E. Pagani, P. Preziosa, M. Filippi, and M.A. Rocca, Measurement of white matter fiber-bundle cross-section in multiple sclerosis using diffusion-weighted imaging. Journa 2020; 1352458520938999. [11] T.E. Nichols, and A.P. Holmes, Nonparametric permutation tests for functional neuroimaging: a primer with examples. Journa 2002; 15: 1-25.Figures
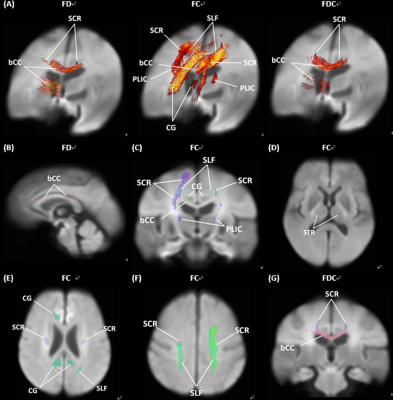
FIG 1. Whole brain fixel-based analysis compared patients with PD-NC to patients with PD-MCI. (A) Significant fixels were colored by family-wise error corrected p values. (B-G) Significant fixels were colored by direction (anterior-to-posterior: green; superior-to-inferior: blue; and left-to-right: red). bCC: body of corpus callosum; CG: cingulum; PLIC: posterior limb of internal capsule; SCR: superior corona radiata; SLF: superior longitudinal fasciculus; STR: superior thalamic radiation.
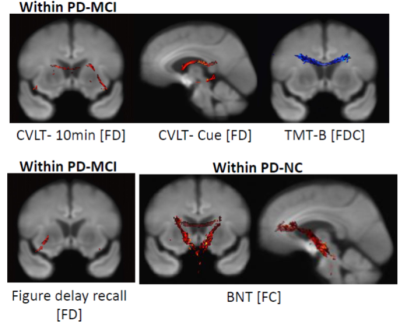
Relationship between the differences in clinical parameters and fixel-based metrics. Fixel-wise regression analysis was performed to identify the associations between clinical parameters and fixel-based metrics. Significant fixels (family-wise error corrected p < 0.05) were colored according to p-values
DOI: https://doi.org/10.58530/2022/3066