3063
Radiomics Molecular Mechanism and Candidate Prognostic Biomarker Investigation for Glioblastoma Multiforme Utilizing WGCNA1Department of Radiology, Liaocheng People's Hospital, Shandong First Medical University & Shandong Academy of Medical Sciences, Liaocheng, China, 2Philips Healthcare, Shanghai, China
Synopsis
In this study, we aimed to explore the deeper molecular mechanism of radiomics features, and uncover new GBM prognostic biomarker. We extracted radiomics features out of 57 patients with MRI data obtained from TCIA database and corresponding clinical and transcription information from TCGA database. WGCNA revealed that the radiomics features are correlated with numerous cancer-associated biological processes, including regulation of vasculature development and so on. HMGA2 as the hub gene was screened by Cytoscape software. There was significant difference in survival analysis between the high and low expression groups, indicating that HMGA2 may be a new prognostic biomarker for GBM.
Introduction
Glioblastoma multiforme (GBM), as the most common malignant tumor in the central nervous system, has the poorest prognosis in clinic nowadays.1With the rapid development of genomic technology, the status of MGMT promoter methylation, 1p and 19q co-deletions, and IDH1/2 mutations, now play major roles in GBM diagnostics and clinical decision making. 2 Radiomics exploits high-throughput features extraction algorithms to extract quantitative imaging features. Tan et al. used MRI radiomics features to predict IDH genotype in astrocytomas with an AUC of 90% in the training cohort and 89% in the validation cohort. 3 However, the correlation between radiomics features and the deeper molecular mechanism is still unclear, and novel molecular and imaging prognostic biomarkers are urgently required to increase overall patient survival.
Therefore, the purpose of this study was to uncover new GBM prognostic biomarker using weighted gene coexpression network analysis (WGCNA), and reveal the correlation between radiomics features and molecular mechanism of GBM, which may provide chances to improve the clinical outcome of GBM patients.
Materials and Methods
Patients’ data collectionTranscription profiles and clinical information (i.e., age, gender, race, year of diagnosis, tumor type, surgical treatment, radiotherapy, chemotherapy, cause of death, and survival time) of 57 GBM patients were downloaded from the Cancer Genome Atlas (TCGA) database. In addition, the corresponding fluid attenuated inversion recovery (FLAIR) MRI data was collected from The Cancer Imaging Archive (TCIA) database.
Data analysis
Pyradiomics software was used to extract quantitative radiomics features. The features screened by the least absolute shrinkage and selection operator (LASSO) Cox regression with ten-fold cross-validation to develop the radiomics signature. A radiomics risk score was calculated for each patient via a linear combination of selected features and weighted according to their respective coefficients. All patients were divided into high-risk group and low-risk group according to the median radiomics risk score.
WGCNA was applied to construct coexpression modules and to detect the mRNA modules related with the radiomics risk score; then, GO/KEGG enrichment analysis was performed to predict the biological function of the interested modules using clusterProfiler package.
Cytoscape version 3.7.1 was used to screen hub gene of the interested modules according to the MCC algorithm. Subsequently, the expression of mRNA level of hub gene was obtained from the Oncomine database, and the survival analysis of hub gene was explored by the OncoLnc database.
Results
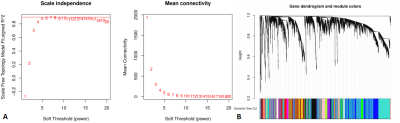
A total of 851 quantitative radiomics features were extracted from FLAIR MRI data. Using LASSO Cox regression analysis, 6 radiomics features were identified. Based on the median radiomics risk score of the patients (median = 1.85), the GBM patients were divided into the high-risk group (n = 28) and low-risk group (n = 29), a significant difference of the overall survival time was found between groups (P < 0.05).WGCNA was used to find gene modules that are significantly correlated with the radiomics risk score (Figures 1A, B). 22 modules of covariant gene sets were identified. Among these modules, the most relevant module is the turquoise module (R = 0.29, P < 0.05) with 129 genes, which was selected for GO/KEGG enrichment analysis. For GO enrichment, angiogenesis was the most significant term. For KEGG enrichment, these genes were significantly enriched in the Rap1 signalling pathway (Figures 2A, B).
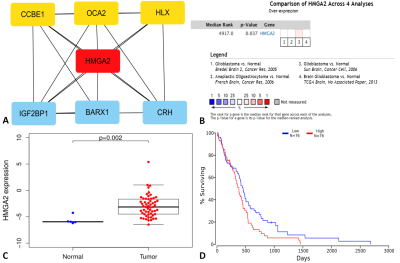
Cytoscape was used to create an interaction network including genes in the turquoise module, and high mobility group AT-hook 2 (HMGA2) was identified as the hub gene through the MCC algorithm (Figures 3A). In a meta-analysis of 4 studies in the Oncomine database, the HMGA2 gene was ranked as 4917.0 in all differentially expressed genes (Figures 3B). The expression of HMGA2 was significantly higher in tumor tissues compared with normal adjacent tissues (P < 0.05) (Figures 3C). Furthermore, survival analysis of GBM patients using the OncoLnc database showed that a high expression of HMGA2 was associated with a poor prognosis (P < 0.05) (Figures 3D).
Discussion and Conclusion
In this study, we extracted radiomics features from FLAIR MRI data of GBM, and subsequently explored the deeper molecular mechanism of radiomics features through co-expression network. The results indicated that the radiomics risk score was significantly correlated with multiple processes of angiogenesis, which have been proved to be associated with tumorigenesis and progression. 4 Finally, we identified HMGA2 as the hub gene through the MCC algorithm. HMGA2 is a DNA binding protein that plays an oncogenic role in GBM and can promote GBM cell clonogenicity, invasion, and tumorigenicity. 5In conclusion, this study revealed that the radiomics features are correlated with many cancer-associated biological processes, and HMGA2 may be used as a new prognostic marker for GBM.
Acknowledgements
Founded by Grant-in-aid for scientific research from the National Natural Science Foundation of China (No. 61976110)References
1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016 [J]. Neuro Oncol. 2019, 21(Suppl 5):v1-v100.
2. Aldape K, Zadeh G, Mansouri S, et al. Glioblastoma: pathology, molecular mechanisms and markers [J]. Acta Neuropathol. 2015, 129(6): 829-848.
3. Tan Y, Zhang S, Wei J, et al. A radiomics nomogram may improve the prediction of IDH genotype for astrocytoma before surgery [J]. European Radiology, 2019, 29(7): 3325-3337.
4. Ahir BK, Engelhard HH, Lakka SS. Tumor Development and Angiogenesis in Adult Brain Tumor: Glioblastoma [J]. Mol Neurobiol. 2020, 57(5): 2461-2478.
5. Kaur H, Ali SZ, Huey L, et al. The transcriptional modulator HMGA2 promotes stemness and tumorigenicity in glioblastoma [J]. Cancer Lett. 2016, 377(1): 55-64.
Figures