3058
Tract-specific association of myelin water fraction with cognitive decline and motor dysfunction in Parkinson’s disease1National Neuroscience Institute, Singapore, Singapore, 2Duke-NUS Graduate Medical School, Singapore, Singapore, 3Nanyang Technological University, Singapore, Singapore, 4Singapore General Hospital, Singapore, Singapore, 5Institue of Bioengineering and Bioimaging, Singapore, Singapore, 6Seoul National University, Seoul, Korea, Republic of
Synopsis
We found associations between tract-specific myelin water fraction and cognitive decline, and motor dysfunction in patients with Parkinson’s disease (PD). Myelin water fractions (MWF) in the anterior corona radiata and posterior thalamic radiations were associated with cognitive decline in PD whilst those in the superior and posterior corona radiata, posterior limb of internal capsule and corticospinal tract were associated with moderate to severe motor dysfunction in PD. Our results suggest that MWF has potential utility as an objective imaging biomarker to monitor longitudinal changes in target WM tracts relevant to motor and cognitive dysfunction in PD neurodegeneration.
Introduction
Diffusion tensor imaging (DTI) has been widely utilised for the non-invasive assessment of white matter (WM) in PD1, but myelin water imaging (MWI) is more specific to changes in myelin. MWI has been shown to detect reduced myelination in multiple sclerosis, neuromyelitis optica and traumatic brain injury.2 Myelin is also postulated to be involved in the pathophysiology of PD due to findings of autoantibodies against oligodendrocytes.3,4 Furthermore, benztropine, an anticholinergic, has been shown to stimulate remyelination in rodent models, possibly contributing to its clinical effectiveness in PD.5 Therefore, myelin specific imaging techniques may be useful in studying PD.We investigated changes in myelin water fraction (MWF) using visualization of short transverse relaxation time component (ViSTa) MWI6 in the basal ganglia-thalamo-cortical circuitry and corpus callosum in relation to motor and non-motor dysfunction.
Methods
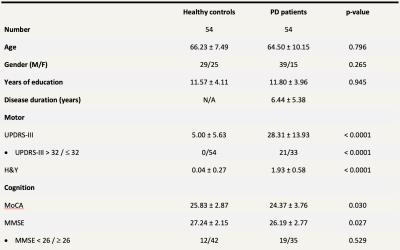
The study is approved by the local institutional ethics board. Fifty-four PD patients and 54 age-matched healthy controls (HC) formed the study population (Table 1). Patients were diagnosed by a movement disorders neurologist with more than 2 decades of practice based on prevailing clinical criteria. All participants underwent motor and cognitive assessments, comprising the Unified Parkinson’s Disease Rating Scale (UPDRS) and Montreal Cognitive Assessment (MoCA), and brain MRI on a 3T scanner. The following MR sequences were acquired: (i) 3D segmented EPI-based ViSTa MWI: TR/TE = 1160/6.8 ms, TI1/TI2 = 560/220 ms, in-plane resolution = 1.4 × 1.4 × 4.0 mm3, number of slices = 32, scan time = 6 min 53 sec, (ii) proton density (PD)-weighted gradient echo (GRE) sequence based on the same EPI as the ViSTa sequence, without the two inversion pulses (TR = 100 ms; FA = 28°; scan time = 35 sec), and (iii) diffusion spectrum imaging: SMS slice factor 3, TR/TE = 4100/110ms, in-plane resolution = 2 × 2 × 2 mm3, number of slices = 81, 128 diffusion samplings with b-values up to 3000 s/mm2, scan duration = 9 min 17 sec.For ViSTa quantification, myelin water fraction (MWF) was calculated by dividing ViSTa data by the PD-weighted GRE data and multiplying it by a scaling factor. The output was referred to as the apparent MWF (aMWF), which was roughly one third of the conventional MWF due to the incomplete scaling factor.6
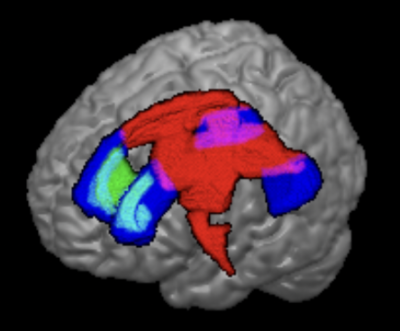
Skeletons of white matter aMWF was generated using TBSS in FSL.7 Mean aMWF values from the basal ganglia-thalamo-cortical circuitry and corpus callosum were extracted using the Johns Hopkins University ICBM-DTI-81 WM atlas.8 The aMWF values of bilateral WM tracts were averaged between hemispheres.
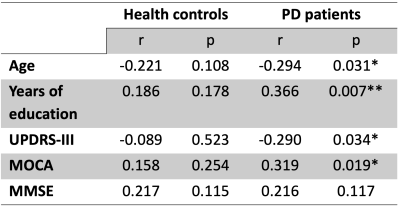
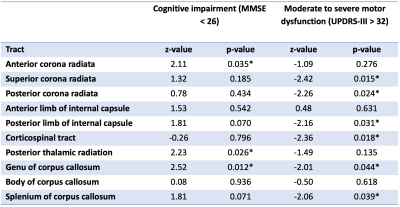
Normality of all clinical parameters and aMWF were determined visually via histograms. Unpaired t-test and Mann-Whitney tests were used to test for differences between normally and non-normally distributed datasets, respectively. Correlation analysis via Pearson’s and Spearman’s tests were performed for normally and non-normally distributed datasets respectively to test the association between aMWF and clinical parameters. Logistic regression using the generalized linear model was performed to determine the utility of aMWF to predict clinical scores after adjusting for age, gender, years of education and the Levodopa equivalent dose. A MMSE score of <26 is used as a cut-off for cognitive impairment9 and a UPDRS-III score of >32 used as a cut-off for moderate to severe motor dysfunction.10 All statistical analysis was performed at significance of p<0.05.
Results
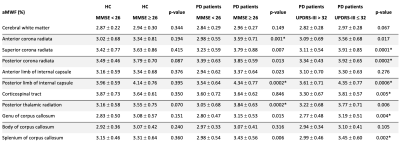
The study demographics are detailed in Table 1. Cerebral WM aMWF were similar between PD (2.92 ± 0.29%) and HC (2.93 ± 0.29%) subjects. In addition, cerebral WM aMWF correlated with age (r = -0.294, p = 0.031), years of education (r = 0.366, p = 0.007), MOCA score (r = 0.319, p = 0.019) and UPDRS-III (r = -0.290, p = 0.034) in PD but not in HC (Table 2).Reduced aMWFs in the anterior corona radiata, posterior thalamic radiation and genu of corpus callosum were associated with cognitive impairment in PD, whilst that in the superior and posterior corona radiata, posterior limb of internal capsule, corticospinal tract, genu and splenium of the corpus callosum were associated with moderate to severe motor dysfunction in PD (Table 3, Figure 1). Post-hoc group comparison (Table 4) showed that myelin water fraction reduction was accentuated in PD patients with cognitive decline compared to HC.
Discussion & conclusion
Cerebral MWF results using ViSTa in our case-control cohort were consistent with a previous study using the more conventional MWI technique (T2 relaxometry with GRASE).11 Findings of associations between aMWF and MOCA were also consistent with previous dementia studies that found an association between myelin breakdown and cognitive decline.12,13Logistical regression analysis demonstrated associations between tract-specific myelin water fraction reduction with cognitive decline (MMSE<26) and motor dysfunction (UPDRS-III>32) in PD patients. Except for the genu of the corpus callosum, WM tracts that were associated with clinical measures of cognitive decline were discrete from those for motor dysfunction. The functional associations of the affected tracts were also congruent with that known in the literature.
Our findings suggest that ViSTa is a viable alternative to GRASE MWI. Its utility as an objective imaging biomarker to study longitudinal changes in target WM tracts relevant to motor and cognitive dysfunction in PD neurodegeneration could be further evaluated in a larger longitudinal cohort.
Acknowledgements
We wish to acknowledge funding support from National Medical Research Council Singapore for this research project.References
1. Atkinson-Clement C, Pinto S, Eusebio A, Coulon O. Diffusion tensor imaging in Parkinson's disease: Review and meta-analysis. NeuroImage Clin 2017;16:98-110.
2. MacKay A, Laule C. Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin. Brain Plasticity. 2016;2(1):71-91.
3. Papuć E, Kurzepa J, Kurys-Denis E, Grabarska A, Krupski W, Rejdak K. Humoral response against glial derived antigens in Parkinson’s disease. Neurosci Lett 2014;566:77–81.
4. Papuć E, Rejdak K. Anti-MAG autoantibodies are increased in Parkinson’s disease but not in atypical parkinsonism. J Neural Transm 2017;124:209–216.
5. Deshmukh V, Tardif V, Lyssiotis C, et al. A regenerative approach to the treatment of multiple sclerosis. Nature. 2013;502(7471):327-332.
6. Oh SH, Bilello M, Schindler M, et al. Direct visualization of short transverse relaxation time component (ViSTa). Neuroimage 2013 Dec;83:485-92.
7. Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage 2006;31:1487-1505.
8. Mori S, Oishi K, Jiang H, et al. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 2008;40(2):570-582.
9. Feng L, Chong MS, Lim WS, Ng TP. The Modified Mini-Mental State Examination test: normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singapore Med J. 2012;53(7):458-62.
10. Martinez-Martin P, Rodriguez-Blazquez C, Alvarez M, et al. Parkinson's disease severity levels and MDS-Unified Parkinson's Disease Rating Scale. Parkinsonism Relat Disord. 2015;21(1):50-4.
11. Baumeister TR, Kim JL, Zhu M, et al. White matter myelin profiles linked to clinical subtypes of Parkinson's disease. J Magn Reson Imaging 2019;50(1):164-174.
12. Bartzokis G, Lu PH, Mintz J. Quantifying age-related myelin breakdown with MRI: novel therapeutic targets for preventing cognitive decline and Alzheimer's disease. J Alzheimers Dis. 2004 Dec;6(6 Suppl):S53-9.
13. Bartzokis G, Lu PH, Geschwind DH, et al. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch Gen Psychiatry 2006 Jan;63(1):63-72.
Figures