3057
Iron Spatial Distributions in Deep Gray Nuclei are Related to Motor Outcomes of Subthalamic Nuclei Deep Brain Stimulation for Parkinson's Disease1East Chian Normal University, Shanghai, China, 2Department of Neurosurgery, Changhai Hospital, Shanghai, China, 3Department of Radiology, Changhai Hospital, Shanghai, China, 4MR Scientific Marketing, Siemens Healthineers, Shanghai, China, 5Department of Radiology, Weill Medical College of Cornell University, New York, NY, United States
Synopsis
The objective of this study was to investigate the relationship between motor outcomes of subthalamic nuclei deep brain stimulation (STN-DBS) and spatial distribution of iron in the deep gray nuclei for patients with Parkinson's disease (PD). The first and second texture parameters were obtained from the preoperative QSM of 40 PD patients. Significant correlations were found between motor improvement after DBS and QSM texture parameters in substantia nigra (SN) and dentate nucleus (DN). Linear regression showed that second-order texture parameter angular second moment in SN (r = -0.449, p = 0.004) had the highest correlation with the STN-DBS motor outcomes.
Introduction
Deep brain stimulation (DBS) of the subthalamic nucleus (STN) improves motor deficits in patients with advanced Parkinson's disease (PD), but improvement outcome varies across individuals 1,2. The clinical predictors of the DBS motor outcome such as the levodopa challenge test, though have been extensively described, are still debated 3. Therefore, objective and unbiased predictive factors for DBS candidate selection are much desired in the pre-surgical process 4–6. The iron deposition has been implicated in the pathogenesis of PD and resulted in spatial heterogeneous microstructural changes in deep gray matter in PD 7. Quantitative susceptibility mapping (QSM) and texture analysis can capture this difference 8–11. In this study, we investigated whether the spatial distribution of iron in the substantia nigra (SN), subthalamic nucleus (STN) and dentate nucleus (DN) from preoperative QSM is useful for selecting PD candidates for STN-DBS.Materials and Methods
This study was approved by the local ethical committee and all participants signed an Informed Consent form. Forty PD patients with a mean age of 63.30±7.45 years old (22 males and 18 females) were recruited in this study. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) 12 scores were first assessed in the off-medication (med_OFF) and on-medication (med_ON) states 1 month before surgery. Postoperative MDS-UPDRS III scores were also obtained in the on-stimulation& off-medication (DBS_ON&med_OFF) state at 6-months follow-up. Motor improvements after DBS were calculated according to the formula:$$Improvment_DBS(%)=(pre_op med_OFF - post_op DBS_ON&med_OFF)/(pre_op med_OFF )
All subjects were scanned on a clinical 3T MRI scanner (GE Signa HDxt) equipped with a 20-channel head coil. Susceptibility maps were generated from a 3D spoiled bipolar-readout multi-echo GRE sequence with the following parameters: TR = 28 ms, TE1 = 4.2 ms, ΔTE = 3.96 ms, number of echoes = 6, flip angle = 12˚, FOV = 240 × 240 mm2, matrix size = 256 × 256, slice thickness = 0.9 mm, number of slices = 160, parallel imaging acceleration factor = 2, voxel size = 0.90 × 0.90 × 0.90 mm3.
QSM images were reconstructed using the Morphology Enabled Dipole Inversion with automatic uniform cerebrospinal fluid zero reference (MEDI+0) algorithm 13. Regions of interest (ROIs), including the bilateral SN, STN, and DN were drawn manually on the QSM images using ITK-SNAP (http://www.itk-snap.org). 3D first- and second-order texture analyses of the segmented ROIs were conducted using MaZda software (http://www.eletel.p.lodz.pl/programy/mazda/, Lodz, Poland). The first-order texture parameter was the mean susceptibility value. Second-order texture parameters included angular second moment (AngScMom), contrast, correlation, difference of variance (DifVarnc), inverse different moment (InvDfMom), entropy, sum of entropy (SumEntrp), difference of entropy (DifEntrp), sum of average (SumAverg), sum of variance (SumVarnc), and sum of squares (SumOfSqs).
Correlations between the motor improvement ratio and features of pre-operative susceptibility maps were assessed using Pearson and Spearman’s correlation coefficients (according to statistical distribution). All p-values reported are two-tailed and the adjusted p-values less than 0.05 were chosen to designate significant correlations. All statistical analyses were carried out using IBM SPSS Statistics 22.
Results
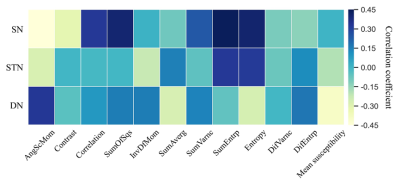
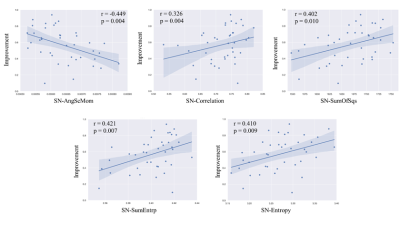
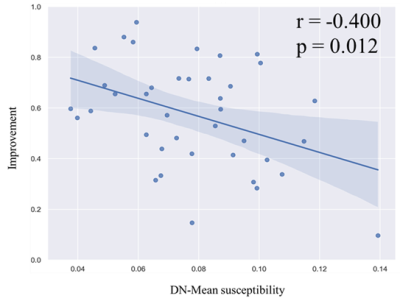
Significant correlations were found between total motor improvement ratio and texture parameters of deep grey matter nuclei (Fig. 1). The DBS outcome was significantly correlated with the AngScMom (r = -0.449, p = 0.004), Correlation (r = 0.326, p = 0.040), SumOfSqs (r = 0.402, p = 0.010), SumEntrp (r = 0.421, p = 0.007) and Entropy (r= 0.410, p = 0.009) of the SN (Fig. 2). In the DN, the mean susceptibility value was significantly negatively correlated with DBS response (r= -0.400, p = 0.012) (Fig. 3). No significant correlations were found between motor improvement outcome and the other texture parameters in the SN, STN and DN (p > 0.5).Discussion
In this study, multiple second-order texture parameters of susceptibility maps within the SN were associated with the clinical motor improvement of STN-DBS in patients with PD. Interestingly, patients with a more homogeneous iron distribution throughout the SN as characterized by AngScMom responded worse to DBS treatment. A more homogeneous iron distribution may reflect clinically more severe motor impairment, which was agreed with a recent study 14. Iron induces oxidative damage through the production of reactive oxygen species and orchestrates “ferroptosis” 15, a recently identified form of iron-mediated cell death, and ferroptosis has been identified in PD patients 16. Uniform neurodegeneration associated with homogeneous iron distribution in SN seems to impede DBS outcome. Possible residual neurons associated with inhomogeneous iron distribution in SN seem to improve DBS outcome.Motor improvement was negatively correlated with the mean susceptibility value in DN. This finding could be explained by that alterations in iron concentration in the DN were correlated with tremor severity in PD and may interrupt normal cerebello-thalamo-cortical (CTC) function 17–19 and thus responded worse to DBS treatment.
Conclusions
In summary, texture features in preoperative susceptibility maps of SN and DN correlate with the motor outcomes of DBS in PD patients. These results provide a new insight into predictive biomarkers of DBS response: iron disposition spatial heterogeneity on QSM may predict the variability of DBS motor improvements and be useful for presurgical patient selection.Acknowledgements
No acknowledgement found.References
1. Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2006;355(9):896-908. doi:10.1056/NEJMoa060281
2. Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2010;362(22):2077-2091. doi:10.1056/NEJMoa0907083
3. Mueller K, Urgošík D, Ballarini T, et al. Differential effects of deep brain stimulation and levodopa on brain activity in Parkinson’s disease. Brain Commun. 2020;2(1):fcaa005. doi:10.1093/braincomms/fcaa005
4. Moro E, Lang AE. Criteria for deep-brain stimulation in Parkinson’s disease: review and analysis. Expert Rev Neurother. 2006;6(11):1695-1705. doi:10.1586/14737175.6.11.1695
5. Fasano A, Daniele A, Albanese A. Treatment of motor and non-motor features of Parkinson’s disease with deep brain stimulation. Lancet Neurol. 2012;11(5):429-442. doi:10.1016/S1474-4422(12)70049-2
6. Hartmann CJ et al. An update on best practice of deep brain stimulation in Parkinson’s disease. Ther Adv Neurol Disord. 2019;12:1756286419838096. doi:10.1177/1756286419838096
7. Massey LA, Miranda MA, Al-Helli O, et al. 9.4 T MR microscopy of the substantia nigra with pathological validation in controls and disease. Neuroimage Clin. 2017;13:154-163. doi:10.1016/j.nicl.2016.11.015
8. Azuma M, Hirai T, Yamada K, et al. Lateral Asymmetry and Spatial Difference of Iron Deposition in the Substantia Nigra of Patients with Parkinson Disease Measured with Quantitative Susceptibility Mapping. AJNR Am J Neuroradiol. 2016;37(5):782-788. doi:10.3174/ajnr.A4645
9. Du G, Liu T, Lewis MM, et al. Quantitative susceptibility mapping of the midbrain in Parkinson’s disease. Movement Disord. 2016;31(3):317-324. doi:10.1002/mds.26417
10. Langkammer C, Pirpamer L, Seiler S, et al. Quantitative Susceptibility Mapping in Parkinson’s Disease. PloS one. 2016;11(9):e0162460. doi:10.1371/journal.pone.0162460
11. Li G, Zhai G, Zhao X, et al. 3D texture analyses within the substantia nigra of Parkinson’s disease patients on quantitative susceptibility maps and R2(∗) maps. NeuroImage. 2019;188:465-472. doi:10.1016/j.neuroimage.2018.12.041
12. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disord. 2008;23(15):2129-2170. doi:10.1002/mds.22340
13. Liu Z et al. MEDI+0: Morphology enabled dipole inversion with automatic uniform cerebrospinal fluid zero reference for quantitative susceptibility mapping. Magn Reson Med. 2018;79(5):2795-2803. doi:10.1002/mrm.26946
14. Liu Y, Xiao B, Zhang C, et al. Predicting Motor Outcome of Subthalamic Nucleus Deep Brain Stimulation for Parkinson’s Disease Using Quantitative Susceptibility Mapping and Radiomics: A Pilot Study. Front Neurosci. 2021;15:731109. doi:10.3389/fnins.2021.731109
15. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060-1072. doi:10.1016/j.cell.2012.03.042
16. Mahoney-Sánchez L, Bouchaoui H, Ayton S, Devos D, Duce JA, Devedjian J-C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog Neurobiol. 2021;196:101890. doi:10.1016/j.pneurobio.2020.101890
17. He N, Huang P, Ling H, et al. Dentate nucleus iron deposition is a potential biomarker for tremor-dominant Parkinson’s disease. NMR Biomed. 2017;30(4). doi:10.1002/nbm.3554
18. Guan X, Xuan M, Gu Q, et al. Influence of regional iron on the motor impairments of Parkinson’s disease: A quantitative susceptibility mapping study. Journal of magnetic resonance imaging : JMRI. 2017;45(5):1335-1342. doi:10.1002/jmri.25434
19. Paris-Robidas S, Brochu E, Sintes M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135(Pt 1):105-116. doi:10.1093/brain/awr301
Figures