3054
Thalamic Plasticity in Brains with Glioma Investigated with Discrete Wavelet Transformation Analysis of Resting-state BOLD Fluctuations1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Huashan Hospital of Fudan University, Shanghai, China
Synopsis
Thalamic plasticity plays an important role in the functional remodeling in brains with glioma. This study investigated the frequency-specific functional alteration of the thalami in the context of gliomas with discrete wavelet transformation and wavelet-energy. Brains with glioma in the left hemisphere featured with amplified inter-thalamus energy deviation for BOLD signals at 15.63-250mHz irrespective of the malignant grades, as compared with healthy controls. Similar analysis revealed no energy changes for the right brain gliomas. These findings suggest a differential thalamic plasticity that is specific to the tumor laterality, and may leverage innovation in the treatment of glioma involving neuromodulation.
Introduction
Thalamic plasticity plays an important role in signifying the progression of glioma and modulating the brain-wide functional reorganization.1,2 Investigation of the thalamic plasticity would further our understanding of functional diaschisis associated with glioma. In this study, we aimed to assess the frequency-specific alteration of thalamic function based on the discrete wavelet transformation (DWT) analysis3 and energy quantification of resting-state BOLD signals.Subjects and Methods
This study was approved by local institutional review board. A total of 96 subjects with histologically confirmed glioma were consecutively included. Out of the 96 tumors, 58 were located in the left hemisphere(LH, 22 females, aged 39.60 ± 12.16 years, WHO II/III/IV 36/14/8) and 38 in right cerebral hemisphere (RH, 18 females, aged 39.90 ± 12.03 years, WHO II/III/IV 30/3/5). None of the tumors involved the thalamus. Rs-fMRI data was acquired using gradient echo-planar imaging sequence (3.0T, Siemens Verio, Germany) with a 12-channel phased array head coil. The major imaging parameters were TR/TE 2000/35 ms, FOV 210 mm × 210 mm, matrix 64×64, 33 slices with thickness of 4.0 mm, 240 volumes. In addition, rs-fMRI data of 35 healthy subjects was acquired as control (HC, 18 females , aged 33.49 ± 7.74 years). The rs-fMRI data were preprocessed by DPABI with the major steps of removing the first 10 volumes, slice timing correction, realignment for head movement correction, nuisance variables regression including Friston 24-parameter motion model and signals of white matter as well as cerebrospinal fluid, spatial normalization to the standard Montreal Neurological Institute space (MNI152) with voxel size resampled to 3 mm x 3 mm x 3 mm, and spatial smoothing by a Gaussian kernel (FWHM = 4 mm). Regions of interest (ROIs) of bilateral thalami were delineated based on the Anatomical Automatic Labeling atlas. The BOLD signal of each thalamic ROI was averaged and decomposed into seven wavelet subbands by DWT analysis with the Daubechies-4 wavelet chosen as the mother wavelet function (Fig. 1). The energy of each decomposed subband was subsequently calculated based on the following formula:$$$E_{j}=\sum_{k=1}^N S_{j}^{2}(k)$$$, where Sj is the BOLD signal of subband-j, k is the time point, and N is the number of timepoints per subband. The interthalamic deviation index (DI) of the wavelet energy was defined based on the following formula: DIj = (LH_Ej - RH_Ej )/(LH_Ej + RH_Ej), where LH_Ej and RH_Ej represent the energy of the subband-j of the left and right thalamus, respectively. Finally, two-sample t-tests were performed to estimate the intergroup differences of DI for each subband energy between groups of HCs, low-grade gliomas (LGGs, WHO II) and high-grade gliomas (HGGs, WHO III and IV). The significance level was set at P < .05 with false discovery rate (FDR) correction.Results
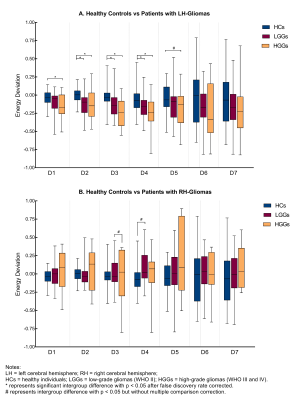
Frequency-dependent thalamic plasticity was identified in brains with glioma based on discrete wavelet transformation analysis. Left brain gliomas were associated with significant energy deviations to the contralesional thalamus for the decomposition subbands 2 to 4 (D2, 62.50~125mHz; D3, 31.25~62.50mHz; D4, 15.63~31.25mHz) as compared to HCs (all p < 0.05, FDR corrected) (Fig. 2A). In particular, left brain HGGs showed significantly larger energy deviation between the bilateral thalami for the decomposition subband-1 (D1, 125~250mHz) as compared to HCs (p = 0.022, FDR corrected). Similar analysis did not find significant changes of thalamic energy for the right brain gliomas (Fig. 2B).Discussion and Conclusions
Alterations of the inter-thalamus wavelet-energy were frequency-dependent and specific to the tumor laterality. The amplified inter-thalamus energy deviation of BOLD signals in patients with left brain glioma ranged from 15.63mHz to 250mHz.The BOLD signals with this frequency range were believed to be associated with the cardiac, respiratory, myogenic, and neurogenic activity.4,5 Therefore, the thalamic plasticity as a result of left brain glioma may be partially mediated by systemic physiological activities in addition to the neurogenic dynamics of the brain. In contrast, similar analysis did not find alteration of the thalamic wavelet-energy for patients with right brain glioma in this study, suggesting a differential thalamic plasticity pattern that is specific to the tumor laterality. These findings provide insights into the pathogenesis of the clinical phenotypes of glioma patients that may not be fully ascribed to the lesion itself, and may leverage innovation in the treatment of glioma involving neuromodulation.Acknowledgements
This study was partially supported by 2021A1515010193, NSFC81627901 and 2020B1212060051.References
1. Li S, Gao L, Liu Y, Ao Y, Xu H. Unilateral Thalamic Glioma Disrupts Large-Scale Functional Architecture of Human Brain During Resting State. Neuropsychiatr Dis Treat. 2019; 15:947-956.
2. Bouwen BLJ, Pieterman KJ, Smits M, Dirven CMF, Gao Z, Vincent AJPE. The Impacts of Tumor and Tumor Associated Epilepsy on Subcortical Brain Structures and Long Distance Connectivity in Patients With Low Grade Glioma. Front Neurol. 2018; 9:1004.
3. Smith RX, Jann K, Ances B, Wang DJ. Wavelet-based regularity analysis reveals recurrent spatiotemporal behavior in resting-state fMRI. Human brain mapping. 2015;36(9):3603-20.
4. Whittaker JR, Driver ID, Venzi M, Bright MG, Murphy K. Cerebral Autoregulation Evidenced by Synchronized Low Frequency Oscillations in Blood Pressure and Resting-State fMRI. Front Neurosci. 2019; 13:433.
5. Laura WMV, Jacobus FAJ , Daan H, et al. Exploring the neurovascular nature of spontaneous cerebral BOLD fluctuations in 1730 individuals-The Maastricht Study. Proc. Intl. Soc. Mag. Reson. Med. 28 (2020); 1363.
Figures