3047
Quantitatively Assessing Longitudinal Alteration of Shear Stiffness in Neonatal Punctate White Matter Lesions based on Virtual Elastography1the Department of Radiology, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Synopsis
Punctate white matter lesions (PWMLs) were currently common white matter injuries, with incidence of 20-30% and associated with adverse neurological development. Longitudinal alteration of PWMLs was less researched which might be related to pathology. Therefore, this study aimed to assess alterations of PWMLs virtual shear stiffness in neonates via DKI-based virtual elastography. We found that virtual shear stiffness of PWMLs was higher than contemporaneous white matter at neonatal periods and did not change obviously with age, while virtual shear stiffness of normal white matter was increased with age. Virtual elastography might be a potential method to identify differently nosological PWMLs.
Introduction
Punctate white matter lesions (PWMLs) were currently common moderate white matter injuries1,2. It was frequently recognized in preterm neonates with incidence of about 20-30%2-4 and also observed in term neonates5, associating with cognitive and motor neurological impairment6,7. At present, there were few studies focused on longitudinal alteration of PWMLs in neonates which might be related to pathology. It was noteworthy that Le Bihan et al. proposed a hypothesis of diffusion MR imaging–based virtual elastography to provide information on the degree of tissue without using mechanical vibrations8.Therefore, this retrospective study aimed to assess alterations of PWMLs virtual shear stiffness in neonates via diffusion kurtosis imaging (DKI) based virtual elastography.Materials and Methods
The Institutional Review Broad of the first author’s affiliation approved this study and written informed consent was obtained from parents of the children.Patients Neonates with PWMLs were included and then were followed up at early childhood. The final included subjects completed conventional MRI and DKI during neonatal period and childhood.
MR Protocols All subjects were examined by using a 3.0T scanner (Signa HDxt, General Electric Medical System, Milwaukee, WI, USA) with an 8-channel head coil. Data acquisition included three-dimensional fast spoiled gradient-echo T1-weighted sequence (TR/TE, 10.2ms/4.6ms; NEX, 1; isotropic 1×1×1mm3; FOV, 24cm) and transverse fast spin-echo T2-weighted sequence (TR/TE, 4200ms/116ms; NEX of 1.5; matrix, 320×320; thickness, 4mm; FOV, 24cm), followed by a DKI (25 directions; b value, 50, 100, 200, 500, 1000, 1500, 2000, 2500 s/mm2; SENSE factor, 2; TR/TE, 11000/93.9ms, slice thickness, 4mm with 4mm gap, FOV, 24cm; matrix, 172×172).
Data and statistical analysis DKI raw data were preprocessed by Matlab and images of b value 200 and 1000s/mm2 were extracted. FMRIB software library (FSL; http://www.fmrib.ox.ac.uk/fsl) was used to correct eddy current of extracted diffusion images. Then diffusionbased tissue shear modulus was calculated from signal intensity attenuation of b200 and b1000 through a custom-designed-automated method. Virtual shear stiffness of PWMLs and anterior corona radiata (ACR) was obtained on a region-of-interest basis at neonatal period and childhood. ACR was regarded as control comparison with PWMLs. Longitudinal virtual shear stiffness of PWMLs and ACR were compared via paired-samples t-test at neonatal period and childhood. Virtual shear stiffness ratio of PWMLs and ACR was used to further explore the alteration during neonatal period and childhood via paired-samples t-test. Then longitudinal virtual shear stiffness alteration of PWMLs and ACR were explored by Spearman-correlation during two periods.
Results
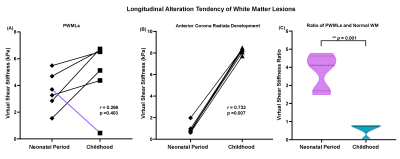
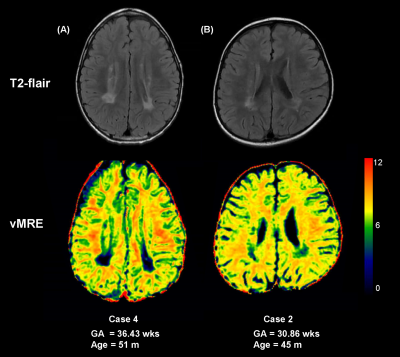
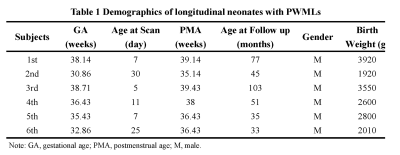
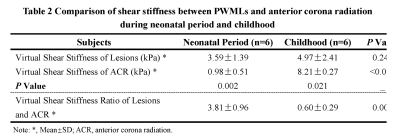
Six neonates with PWMLs were included and were followed up at childhood aged 33 months to 103 months completing twice MRI examinations. Demographics were showed at table 1. Longitudinal alteration of PWMLs virtual shear stiffness was not significantly different between neonatal period and childhood (μ_stiff, 3.59±1.39 vs. 4.97±2.41, p=0.246; r=0.266, p=0.403) (Figure 1, Table 2). But longitudinal alteration of ACR virtual shear stiffness showed obviously correlation with age (r=0.732, p=0.007) and significantly different between two periods (μ_stiff, 0.98±0.51 vs. 8.21±0.27, p<0.001) (Figure 1, Table 2). Additionally, different virtual shear stiffness was observed between PWMLs and ACR either neonatal period or early childhood (neonatal period, p=0.002; childhood, p=0.021) (Table 2). Virtual shear stiffness ratio of PWMLs and ACR was used to further explore the alteration during neonatal period and childhood, and it was different at two periods (p=0.001, Figure 1C). Of the 6 neonates with PWMLs, the 4th subject showed different virtual shear stiffness alteration from others. The virtual shear stiffness of lesions was decreased at early childhood while other subjects showed increased stiffness. However, appearance of lesions presented on T2-flair cannot be visually distinguished differences of the 4th subject from others (Figure 2).Discussion
In this study, we found that stiffness of PWMLs was higher than contemporaneous white matter at neonatal periods, while lower at early childhood. And stiffness of brain white matter was increased with age. It can be interpreted as PWMLs mainly represented activated and increased microglia9, and thus higher cells density result in increased stiffness at neonatal period comparing with unmyelinated white matter. With brain development from neonatal to children’s periods, white matter fibers underwent bundle alignment and myelination10, and the stiffness was reasonable increased. Nevertheless, since microglia of neonatal PWMLs didn’t have developmental trajectories as normal white matter, stiffness of PWMLs were not changed obviously at early childhood comparing with neonatal period (p=0.246). Thus, alteration of virtual shear stiffness ratio was due to the normal white matter development, rather than changes of PWMLs. Of the 6 neonates with PWMLs, the 4th subject showed different virtual shear stiffness alteration from others. Virtual shear stiffness of lesions was decreased at early childhood while other subjects showed increased stiffness. It may indicate the different pathology or pathogenesis of PWMLs. Hence, vMRE might be a potential method to identify differently nosological PWMLs. However, currently, our virtual elastography of developing brain was an exploratory research, that the theory was originated from hypothesis in liver8. The relationships of real MRE and virtual elastography in brain were needed to further study.Conclusion
Virtual shear stiffness of white matter was increased with age, while stiffness of PWMLs was higher than contemporaneous white matter at neonatal periods and did not change obviously with age. vMRE might be a potential method to identify differently nosological PWMLs.Acknowledgements
This study was supported by National Natural Science Foundation of China (81901516, 81901823, 81971581, 81771810 and 51706178), Shaanxi Provincial Innovation Team (2019TD-018), National Key Research and Development Program of China (2016YFC0100300).
* Correspondence: Jian Yang, Ph.D., Professor Department of Radiology The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China E-mail: yj1118@mail.xjtu.edu.cn
References
1. Volpe JJ. Confusions in Nomenclature: "Periventricular Leukomalacia" and "White Matter Injury"-Identical, Distinct, or Overlapping? Pediatr Neurol. 2017;73:3-6. 2. de Bruine FT, van den Berg-Huysmans AA, Leijser LM, et al. Clinical implications of MR imaging findings in the white matter in very preterm infants: a 2-year follow-up study. Radiology. 2011;261(3):899-906. 3. Kersbergen KJ, Benders MJ, Groenendaal F, et al. Different patterns of punctate white matter lesions in serially scanned preterm infants. PLoS One. 2014;9(10):e108904. 4. Wagenaar N, Chau V, Groenendaal F, et al. Clinical Risk Factors for Punctate White Matter Lesions on Early Magnetic Resonance Imaging in Preterm Newborns. J Pediatr. 2017;182:34-40 e31. 5. Hayman M, van Wezel-Meijler G, van Straaten H, Brilstra E, Groenendaal F, de Vries LS. Punctate white-matter lesions in the full-term newborn: Underlying aetiology and outcome. Eur J Paediatr Neurol. 2019. 6. Guo T, Duerden EG, Adams E, et al. Quantitative assessment of white matter injury in preterm neonates: Association with outcomes. Neurology. 2017;88(7):614-622. 7. Tusor N, Benders MJ, Counsell SJ, et al. Punctate White Matter Lesions Associated With Altered Brain Development And Adverse Motor Outcome In Preterm Infants. Sci Rep. 2017;7(1):13250. 8. Le Bihan D, Ichikawa S, Motosugi U. Diffusion and Intravoxel Incoherent Motion MR Imaging-based Virtual Elastography: A Hypothesis-generating Study in the Liver. Radiology. 2017;285(2):609-619. 9. Rutherford MA, Supramaniam V, Ederies A, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology. 2010;52(6):505-521. 10. Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48-71.Figures