3044
The value of diffusion kurtosis imaging in detecting delayed brain development of premature infants1Department of Radiology, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2Henan Key Laboratory of Child Brain Injury, Institute of Neuroscience and Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 3McGill university Montreal, Zhengzhou, China, 4GE Healthcare, MR Research China, Beijing, China, 5Wuhan University, Wuhan, China, 6The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Synopsis
Preterm infants are at high risk of adverse neurodevelopmental outcome. Therefore, it is necessary to find a tool to detect brain developmental abnormalities earlier. Diffusion kurtosis imaging (DKI) is considered as a more accurate technology based on a kurtosis model accounting for the variation of Gaussian distribution caused by complex cellular environments in compare with diffusion tensor imaging. The mean kurtosis (MK) in posterior limbs of the internal capsule (PLIC), anterior limb of internal capsule (ALIC), the mean diffusion (MD) in parietal white matter (PWM) were found with good diagnostic performance for abnormal brain microstructural change.
Introduction
In recent years, the birth rate of premature babies has increased significantly in many countries[1]. Compared with full-term babies, the brain developments of premature babies could be impaired due to the young gestational age (GA) [2]. It’s reported that changes caused by abnormal brain development (decreased synaptic density, delayed myelination, and decreased cell connectivity) can affect the diffusion rate and pattern of water molecules in brain tissue [3]. Complex structural changes make the water diffusion present anisotropic. DKI is a magnetic resonance imaging method that can characterize the diffusion of water molecules. Compared with the DTI method, DKI is based on a non-Gaussian diffusion model, which is believed to better reflect the heterogeneous changes in the real brain microstructure [4]. In recent years, DKI has been used to evaluate brain development. research has shown that DKI shows potential advantages in detecting normal brain development in children [5], but few studies have focused on brain development in premature babies, which may help understand the early neurodevelopmental characteristics of premature babies. In this study, we aimed to discusses the value and advantages of DKI in evaluating the brain development of preterm infants with multiple parameters.Material and Methods
In this study, 52 preterm infants including 26 premature infants and 26 full-term infants. All MRI scan were carried out on 3.0 T MR scanner (Pioneer, GE Healthcare, Milwaukee, WI) with T1-weighted imaging (T1WI) Flair, T2-weighted imaging (T2WI) Flair, Diffusion weighted (DWI) imaging and DKI (TR= 2000 ms, TE = 2.32 ms, Directions=15 per b value, b value =0, 1000, 2000 mm²/s). The mean kurtosis (MK), radial kurtosis (RK), fractional anisotropy (FA), mean diffusion (MD) maps were generated from DKI images on vendor-supplied post-processing workstation. Two radiologists outlined 7 regions of interest (ROI) including posterior limbs of the internal capsule (PLIC), anterior limb of internal capsule (ALIC), genu of the corpus callosum (GCC), parietal white matter (PWM), frontal white matter (FWM), thalamus (TH), lenticular nucleus (LN) manually three times respectively and calculated to get the average in each ROI. Student's t test was used to compare DKI parameters between groups. Receiver operating characteristic curve (ROC) was used to evaluate the performance of differentiating the delayed development of premature infants. Spearman’s correlation analysis was used to analyze the correlation between DKI parameters and postmenstrual age (PMA). P<0.05 indicated statistical significance.Results
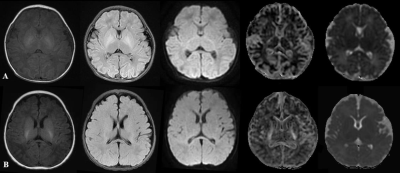
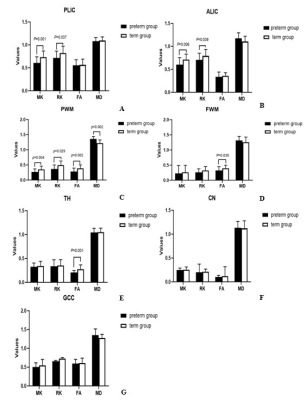
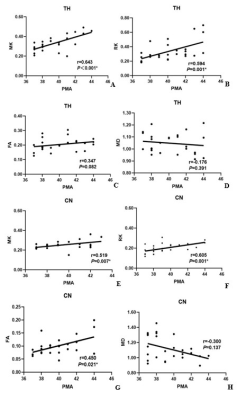
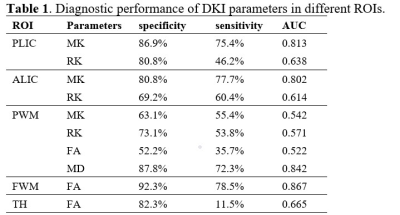
Images including T1WI Flair, T2WI Flair, DWI, MK and MD were displayed in Figure 1 images of two representative newborns. Row A represented: male, GA 30 weeks, PMA 42 weeks. Row B represented: male, GA 37 weeks, PMA 43 weeks. As shown in Figure 2, significant lower values of the MK and RK values in PLIC, PWM and ALIC, and FA values in FWM and TH, and increased MD values (P<0.05 ) in PWM were found between preterm infants and term infants. The ROC analysis (Table 1) showed good diagnostic performance of MK in PLIC (AUC = 0.813, sensitivity =75.4%, specificity =86.9%) and ALIC (AUC =0.802, sensitivity =77.7%, specificity =80.8%), and MD in PWM (AUC =0.842, sensitivity =72.3%, specificity =87.8%) for preterm infants differentiation. As shown in Figure 3, good correlation were observed between MK (r =0.643, P<0.05)/RK (r =0.594, P<0.05) in TH and PMA, MK (r = 0.519, P<0.05)/ RK (r = 0.605, P<0.05) in CN and PMA.Discussion and conclusion
The parameters such as MK and RK from DKI imaging based on non-Gaussian diffusion modelling have been recognized as more comprehensive characterization for revealing the microstructural variation, especially in brain with more complex or heterogeneous tissue. The lower MK and RK values in PLIC and ALIC of the preterm infants may mean that the decreased density of cells and axon membranes [6]. The diffusion of water molecules actually deviates from the normal distribution, so MK can quantify this deviation and RK reflects the limited radial diffusion [7]. The increased MD in PWM can be related with water diffusion increases after brain cells are damaged [8]. The kurtosis parameters MK in PLIC and ALIC showed good diagnostic ability for brain developmental disorders. Because the internal capsule acts as a white matter plate connecting the upper and lower fibers of the cerebral cortex to the brainstem, the structure is more complicated [7]. For this complex structure, MK is more sensitive to the degree of water limited diffusion. The parameters MK and RK in PLIC and ALIC could be used as potential imaging markers for the diagnosis of brain developmental disorders in premature infants.Acknowledgements
The National Natural Science Foundation of China (grant 81870983).References
1.Ushida T, Kidokoro H, Nakamura N, Katsuki S, Imai K, Nakano-Kobayashi T, Moriyama Y, Sato Y, Hayakawa M, Natsume J et al: Impact of maternal hypertensive disorders of pregnancy on brain volumes at term-equivalent age in preterm infants: A voxel-based morphometry study. Pregnancy hypertension 2021, 25:143-149.
2.Baldoli C, Scola E, Della Rosa P, Pontesilli S, Longaretti R, Poloniato A, Scotti R, Blasi V, Cirillo S, Iadanza A et al: Maturation of preterm newborn brains: a fMRI-DTI study of auditory processing of linguistic stimuli and white matter development. Brain structure & function 2015, 220(6):3733-3751. 3.Back S: White matter injury in the preterm infant: pathology and mechanisms. Acta neuropathologica 2017, 134(3):331-349.
4.McKenna F, Babb J, Miles L, Goff D, Lazar M: Reduced Microstructural Lateralization in Males with Chronic Schizophrenia: A Diffusional Kurtosis Imaging Study. Cerebral cortex (New York, NY : 1991) 2020, 30(4):2281-2294.
5.Shi J, Yang S, Wang J, Huang S, Yao Y, Zhang S, Zhu W, Shao J: Detecting normal pediatric brain development with diffusional kurtosis imaging. European journal of radiology 2019, 120:108690.
6.Mukherjee P, Miller J, Shimony J, Philip J, Nehra D, Snyder A, Conturo T, Neil J, McKinstry R: Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR American journal of neuroradiology 2002, 23(9):1445-1456.
7.Olivieri B, Rampakakis E, Gilbert G, Fezoua A, Wintermark P: Myelination may be impaired in neonates following birth asphyxia. NeuroImage Clinical 2021, 31:102678.
8.Das S, Wang J, Bing L, Bhetuwal A, Yang H: Regional Values of Diffusional Kurtosis Estimates in the Healthy Brain during Normal Aging. Clinical neuroradiology 2017, 27(3):283-298.
Figures