3042
Quantifying the Stiffness of Peritrigonal Terminal Zones in Preschool Children by Diffusion Imaging-based Virtual MRI Elastography
Na Zhang1, Xianjun Li1, Congcong Liu1, Yao Ge1, Yuying Feng1, Pengxuan Bai1, Miaomiao Wang1, and Jian Yang1
1Department of Radiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi'an, China
1Department of Radiology, The First Affiliated Hospital of Xi’an Jiaotong University, Xi'an, China
Synopsis
The high signal intensity on T2-weighted images in terminal zones (TZ) mainly associated with lower maturational degree. We hypothesized that the stiffness in these regions may be different from typical developing children without TZ. Virtual stiffness of brain can be measured safely with virtual magnetic resonance elastography (vMRE). Therefore, this study tried to use vMRE to investigate differences between children with and without TZ. Results demonstrated that virtual stiffness of TZ was lower than controls, while higher than periventricular leukomalacia. These suggest that vMRE may provide additional information for evaluating brain maturation and injury.
Introduction
The myelination process complies with specific regulation, which begins at the level of the cranial nerves during the fifth month of intrauterine life, and development continues in postnatal life. [1] The TZ are considered as the last mature region, which is defined as a triangular region posterior and superior to the trigones of the lateral ventricles with high signal intensity on T2-weighted images.[2] However, the recognition for terminal zones is limited. Virtual stiffness of brain can be measured safely with virtual magnetic resonance elastography (vMRE). We hypothesized that the stiffness in these regions may be different from typical developing children without TZ. Virtual stiffness of brain can be measured safely with virtual magnetic resonance elastography (vMRE). In addition, the relation of stiffness between TZ and PLV is not clear. In this study, we aimed to explore the virtual stiffness of brain with TZ and PVL by using vMRE.Materials and Methods
Patients A total of 28 children were enrolled in the study, 13 children with typical terminal zones, 12 controls and 3 children with PVL. All these children completed scan of conventional and diffusion kurtosis imaging (DKI) sequences. TZ is defined as posterior white matter T2 hyperintensity which tend to be slightly higher intensity than the surrounding white matter; hazy, indistinct borders; and no local atrophy.[3] MR Protocols All subjects were examined by using a 3.0T scanner (Signa HDxt, General Electric Medical System, Milwaukee, WI, USA) with an 8-channel head coil. Data acquisition included three-dimensional fast spoiled gradient-echo T1-weighted sequence (TR/TE, 10.2ms/4.6ms; NEX, 1; isotropic 1×1×1mm3; FOV, 24cm) and transverse fast spin-echo T2-weighted sequence (TR/TE, 4200ms/116ms; NEX of 1.5; matrix, 320×320; thickness, 4mm; FOV, 24cm), followed by a DKI (TR/TE, 4200ms/116ms; NEX of 1.5; matrix, 320×320; thickness, 4mm; FOV, 24cm), followed by a DKI (b values = 0, 50, 200, 500, 1000, 2000, 2500 s/mm2; 18 gradient directions per nonzero b value; NEX = 1; repetition time/echo time = 11000/91.7 ms; slice thickness = 4 mm; field of view = 180 × 180 mm2; acquisition matrix =128 × 128; the acquisition voxel size = 1.4 × 1.4 × 4 mm3). Data and statistical analysis Diffusion weighted images of the lower b-value (Slow, b value = 200 s/mm2) and those of the higher b-value (Shigh, b value = 1000 s/mm2) were used to estimate the virtual shear stiffness [4,5]: virtual stiffness = a·ln (Slow/Shigh) + b. The scaling (a) and the shift (b) factors were separately set to −9.8 and 14 according to the previous calibration studies [4,5]. The stiffness was obtained within the region of interest (ROI) drawn by using the software imageJ, and the ROI of TZ and control was at the trigones of the lateral ventricles. We measured 6 ROI in different slice at the lesion of every children with PVL. Kruskal-wallis test were used for the differences in stiffness between three groups. p<0.017 was considered as statistically significant difference.Results
A total of 13 children with TZ, 12 controls, 3 children with PVL at age of 3-5 years old were included. No significant differences in GA, age at MRI scan were found between groups (Table). virtual stiffness of TZ was lower than controls, while higher than PVL. (Table)(Figure)Discussion
As formation of myelin by the oligodendrocytes proceeds, an increase in brain cholesterol and glycolipids concentration and a decrease in water content take place.[6] As a safe, noninvasive method for evaluating the virtual stiffness of brain tissue, vMRE provided us more information about the stiffness of TZ. And it could be used to evaluate the development of the brain. As the results show, PVL is softer than TZ. PVL is characterized by the death of the white matter of the brain due to softening of the brain tissue. The tissue of PVL with less cell was much looser. The result complied with the regulation. We proved it preliminary.Conclusions
The vMRE showed the virtual stiffness can be used to evaluate the development and injure of brain. The truth that the stiffness of PVL is lower than TZ was preliminary proved.Acknowledgements
This study was supported by National Natural Science Foundation of China (81901516, 81901823, 81971581 and 82101815), Shaanxi Provincial Innovation Team (2019TD-018).* Correspondence:JianYang, Professor. Department of Radiology The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China E-mail: yj1118@mail.xjtu.edu.cnReferences
- McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo E G. Developmental features of the neonatal brain at MR imaging: gray-white matter differentation and myelination. Radiology 1987;162:223–229
- Liauw L, van der Grond J, Slooff V, Wiggers-de Bruine F, Laan L, le Cessie S, van Buchem M, van Wezel-Meijler G. Differentiation between peritrigonal terminal zones and hypoxic-ischemic white matter injury on MRI. Eur J Radiol. 2008 Mar;65(3):395-401. doi: 10.1016/j.ejrad.2007.04.016. Epub 2007 May 29. PMID: 17537605.
- Meyers CW, Berg MJ. Benign MRI findings and their pathologic mimics. Neurol Clin Pract. 2013 Apr;3(2):155-160. doi: 10.1212/CPJ.0b013e31828d9f02. PMID: 29473589; PMCID: PMC5765951.
- Le Bihan D, Ichikawa S, Motosugi U. Diffusion and intravoxel incoherent motion MR imaging-based virtual elastography: a hypothesis-generating study in the liver. Radiology, 2017, 285(2):609–619.
- Lagerstrand K, Gaedes N, Eriksson S, et al. Virtual magnetic resonance elastography has the feasibility to evaluate preoperative pituitary adenoma consistency. Pituitary, 2021, 24:530–541.
- McArdle CB, Richardson CJ, Nicholas DA, Mirfakhraee M, Hayden CK, Amparo E G. Developmental features of the neonatal brain at MR imaging: gray-white matter differentation and myelination. Radiology 1987;162:223–229
Figures
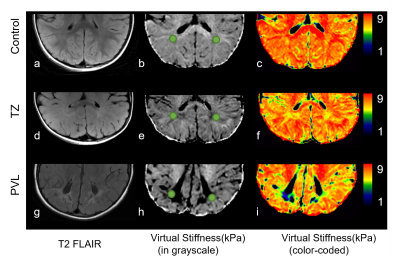
Figure Comparison of virtual stiffness across control,
TZ and PVL. The a-c images from a 5 year-old boy without abnormality; d-f image
form a 4 year-old boy with TZ; g-i images from a 3 year-old girl with PVL; b, e, h images showed the ROI.
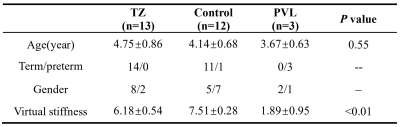
Table Comparisons of demographics and
virtual stiffness across children with TZ, PVL and control.
DOI: https://doi.org/10.58530/2022/3042