3032
Malignancy-specific structural-functional dyscoupling in brains with glioma1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Huashan Hospital of Fudan University, Shanghai, China
Synopsis
The structural-functional coupling in the connectivity of the contralesional hemisphere was enhanced and specific to the malignancy grades of glioma. Structural and functional networks in brains with low grade glioma featured with increased global and local efficiency, whereas the plasticity of the functional networks was limited in brians with high grade glioma. These findings provide new knowledge toward innovating the characterization of glioma.
Introduction
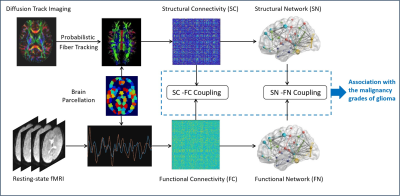
Alteration of structural-functional coupling in the connectivity and network topology is associated with the functional impairment in diseased brain.1,2 However, alterations of the functional-structural coupling in brains with glioma remain less investigated. In this study, we aimed to explore the structure-function coupling in the connectivity and network topology in brains with glioma by quantifying intermodal correlation in the contralesional hemisphere using the diffusion tract imaging (DTI) and resting-state functional MRI (rs-fMRI) data, and contemplate its association with the malignancy grades of gliomas (Fig. 1).Subjects and Methods
This study was approved by local institutional review board. A total of 41 subjects with histologically confirmed glioma in the left hemisphere were consecutively included. Out of the 41 tumors, 13 tumors were classified as WHO II ( 8 females, age = 36.80 ± 14.20 years), 10 as WHO III ( 5 females, age = 37.88 ± 12.86 years), and 18 as WHO IV ( 7 females, age = 49.19 ± 11.73 years). All MRI scans were performed on a 3.0T magnetic scanner (Siemens Verio, Germany) with a 12 channel phased array head coil. DTI was obtained using a echo-planar imaging (EPI) sequence with TR/TE 7,600/91 ms, FOV 230mm × 230 mm, matrix size 128 × 128, slice thickness 3 mm, 20 diffusion gradient encoding directions at b value of 1,000s/mm2. Resting-state BOLD fMRI was acquired using a gradient-echo EPI sequence with TR/TE 2,000/35ms, FOV 210mm × 210mm, matrix 64 × 64, 33 slices with thickness of 4.0mm, 240 volumes. In addition, diffusion and function MRI data of 18 healthy subjects was acquired as controls (HC, 9 females, aged 50.78 ± 6.65 years). PANDA was utilized to preprocess DTI images, conduct the probabilistic fiber tracking, and construct the structural network. The brain was segmented into 392 regions of interest (ROIs) based on a fine-grained atlas (CC400).3 Only the 162 ROIs in the contralesional hemisphere were taken into account. The normalized structural connectivity (SC) between ROIs was defined based on the following fomula2: $$$SC_{ij}=\frac{log(p_{ij})-min[log(p_{mn})]}{max[log(p_{mn})]-min[log(p_{mn})]}$$$, where Pij represents the connection probability between ROI-i and ROI-j, 1 ≤ m ≠ n ≤ N, and N = 162. DPABI was used to preprocess fMRI data and calculate the pairwise functional connectivity (FC) which was defined as the Pearson’s correlation coefficient between the contralesional ROIs with Fisher’s Z transformation. Then, the integrals of the global (Eg) and local efficiency (Eloc)4 across a wide sparsity range (0.1 ≤ sparsity ≤ 0.34, interval = 0.01) were used to quantify the integration and segregation capability of the unsigned structural (SN) and functional networks (FN). Subsequently, the Spearman-rank correlation between non-zero SCs and corresponding FCs was calculated at the individual level. The effects of left-brain gliomas on the contralesional SC-FC coupling and network topological features were estimated with the one-way ANOVA analysis. The significance level was set at P < 0.05 with false discovery rate correction.Results
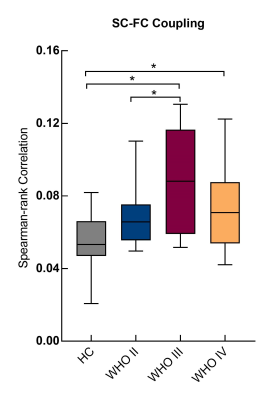
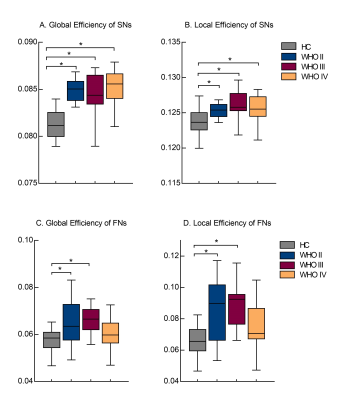
Enhanced SC-FC coupling and network topology were identified in the contralesional hemisphere. The contralesional SC-FC coupling was significantly increased in brains with left-brain WHO III and IV gliomas, as compared to HCs (Fig. 2) (P < 0.05). Brains with WHO III showed significantly higher SC-FC coupling than those with WHO II (P = 0.03). Higher global and local efficiency of SNs was identified for all patients (P < 0.05) (Fig. 3A-B), but only in FNs for patients with WHO II and III, as compared to HCs (P < 0.05) (Fig. 3C-D).Discussion and Conclusions
Increased structural and functional coupling was associated with deteriorated cognitive impairment.2 The enhanced SC-FC coupling of the contralesional hemisphere in this study suggests a functional dysregulation of the brain in addition to the structural damage as a result of tumor growth, emphasizing the rationality to take the SC-FC coupling into account for the investigation of plastic mechanism in brains with glioma. Increased structural and functional network topological efficiency in patients with low-grade gliomas may indicate an intrinsic mechanism featuring enhanced funcitonal integration and segregation to compensate for the tissue loss and functional disturbance in the tumor hemisphere.5 As compared with the structural plasticity, the functional remodeling was limited for patients with high-grade glioma, suggesting the different plasticity potential of structural and functional networks.1 The diversity in the structural-funcitonal coupling provide new insights into the plastic remodling of diseased brain, and may serve as a new biomarker for better characterization of glioma.Acknowledgements
This study was partially supported by 2021A1515010193, NSFC81627901 and 2020B1212060051.References
1. Wang Z, Dai Z, Gong G, Zhou C, He Y. Understanding structural-functional relationships in the human brain: a large-scale network perspective. Neuroscientist. 2015; 21(3):290-305.
2. Wang J, Khosrowabadi R, Ng KK, et al. Alterations in Brain Network Topology and Structural-Functional Connectome Coupling Relate to Cognitive Impairment. Front. Aging Neurosci. 2018; 10:404.
3. Craddock RC, James GA, Holtzheimer PE 3rd, Hu XP, Mayberg HS. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp. 2012; 33(8):1914-28.
4. Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001; 87(19):198701.
5. De Baene W, Rutten GM, Sitskoorn MM. Cognitive functioning in glioma patients is related to functional connectivity measures of the non-tumoural hemisphere. Eur J Neurosci. 2019;50(12):3921-3933.
Figures