3027
Thalamic Reticular Nucleus Exerts Long-range Cross-modal Modulation on Sensory Processing Depending on Inputs?1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
Thalamic reticular nucleus (TRN) has been shown to gate sensory thalamo-cortical interactions and selectively modulate thalamic sensory information processing according to behavioral demands. However, whether TRN can exert long-range, i.e., beyond thalamus, cross-modal modulation of sensory processing remains unclear. In this fMRI study, we demonstrate that optogenetic excitation of somatosensory-specific TRN enhances cross-modal excitatory sensory inputs but suppresses cross-modal competing inputs at somatosensory cortices. Our work provides insight into how TRN differentially gate the processing of distinct cross-modal sensory information at large-scale, which may be critical for ensuring the balance between various task demands.
Purpose
Selective processing of cross-modal sensory information is a fundamental brain function crucial for survival1,2. For example, rodents must be able to balance between all senses for foraging and predator detection. The thalamic reticular nucleus (TRN), which solely consists of inhibitory neurons, is known to provide dynamical control upon excitatory thalamo-cortical neurons of sensory thalamic nuclei to mediate such selective processing of sensory inputs3-7. TRN exhibits topographically-segregated subdivisions with specific projections to each sensory thalamic nuclei to enable modulation of corresponding local sensory cortical regions4,6,7. Research also highlighted that some TRN neurons exhibit cross-modal projections or exert cross-modal sensory gating to sensory thalamus5,8-11. However, it remains unknown whether modality-specific TRN subdivisions can exert long-range modulation on cross-modal sensory processing beyond thalamus and if so where at the whole-brain level. In this study, we combined fMRI with optogenetic and sensory task-based stimulations to interrogate the large-scale modulation effects of modality-specific TRN excitation (e.g., somatosensory-specific) on cross-modal (e.g., cross visual-somatosensory) sensory processing.Methods
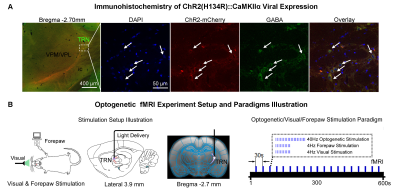
Animal preparation and MRI experimental setup: 3μl rAAV5-hsyn-hChR2(H134R)-mcherry-WPRE-PA was injected to the somatosensory subdivision of TRN6 in adult SD rats. After 4 weeks (Figure 1A), opaque optical fiber cannulas were implanted at the injection sites. All fMRI experiments were performed under 1.0% isoflurane. MRI data was acquired at 7T using GE-EPI.Optogenetic, visual and somatosensory fMRI experiments: Seven stimulation paradigms were employed: optogenetic, visual or forepaw only and combined forepaw with optogenetic stimulations for TRN modality-specific control experiments; combined visual with optogenetic, paired visual and forepaw and combined visual and forepaw with optogenetic stimulations to study TRN cross-modal sensory processing. All stimulations were presented once every 30s (Figure 1B). For optogenetic stimulations, 400 blue light pulses at 40Hz with 10ms pulse width were presented at TRN (473nm; 40mW/mm2). For trials with visual stimulation, binocular visual stimuli (5s, 4Hz, 10% duty cycle; 0.5mW) were presented. For forepaw stimulation, two electrodes were subcutaneously inserted into the right/ipsilateral forepaw: one between the first and second digits and the other between the third and fourth digits. Electrical stimulation was performed via a constant voltage stimulator (5s, 4Hz square wave, 3ms pulse width, 8V). Coherence analysis12 was applied to identify significant BOLD responses.
Results
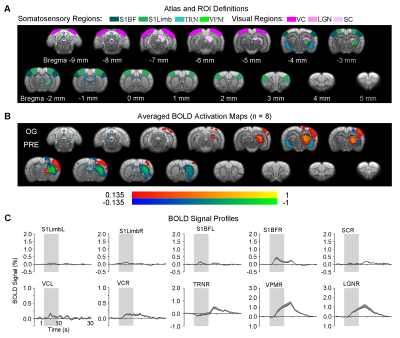
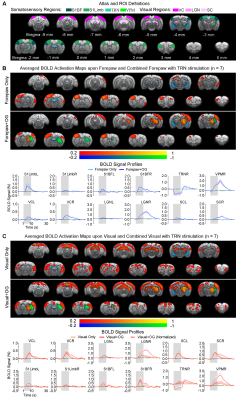
Brain-wide responses driven by inhibitory somatosensory TRN neurons: Optogenetic stimulation of TRN at 40Hz evoked robust positive BOLD responses mainly in ipsilateral sensorimotor cortical and thalamic regions (Figure 2), including somatosensory (primary: barrel field S1BF; limb region S1Limb; upper lip region S1ULp; secondary: S2) and visual (VC) cortices, and somatosensory (ventroposteromedial, VPM) and visual (LGN) thalamus. Negative BOLD responses were evoked at ipsilateral caudate putamen (CPu). Interestingly, TRN and VPM showed biphasic BOLD responses (initial negative and later positive), while sensory cortices showed positive responses only.Somatosensory TRN stimulation suppresses forepaw responses and enhances visually-evoked cross-modal responses at somatosensory cortex: We observed that optogenetic excitation of somatosensory TRN decreased contralateral S1Limb and S1BF responses to forepaw stimuli (Figure 3A), demonstrating the somatosensory-specific TRN modulation effect. Note that the enhanced responses in ipsilateral VPM, S1BF, bilateral VC and SC were due to the optogenetically-evoked responses. In order to examine cross-modal TRN modulation effects on visual responses only, we removed/normalized such somatosensory modality-specific effects and optogenetically-evoked responses. After normalizing the visually-evoked responses by the somatosensory modality-specific effects, no TRN modulation effects were found in VC, LGN and SC, with only the visually-evoked cross-modal S1BF responses that remain elevated (Figure 3B).
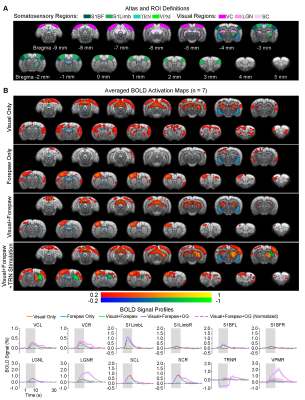
Somatosensory TRN stimulation does not affect somatosensory influences on visual responses but cancels visual influences on forepaw responses (Figure 4): With paired visual and forepaw stimulation, we established that both cortical visual and somatosensory responses (VC and S1Limb) were suppressed, reflecting a cross-modal sensory competition phenomenon. Importantly, the addition of somatosensory TRN stimulations showed no TRN modulation on such cross-modal competition effects at VC (i.e., normalized BOLD responses). However, the suppression effect of visual input to S1Limb forepaw response was canceled, bringing S1Limb response back to the forepaw only stimulation level.
Discussion and Conclusion
Our results demonstrated that the optogenetic stimulation of somatosensory TRN exerted long-range modulation on cross-modal visual processing in somatosensory cortices (i.e., S1Limb, S1BF), but not along the visual pathway (i.e., SC, LGN and VC). Specifically, upon presentation of visual stimulus with somatosensory TRN stimulation, visually-evoked responses at S1BF, not VC/LGN/SC were elevated. Further, under the condition of visual and forepaw cross-modal sensory competition, optogenetic excitation of somatosensory TRN reversed the suppression effects of competing visual input to S1Limb forepaw responses. These findings suggest that TRN modulation of multisensory interactions between sensory cortices is mainly exerted within corresponding pathway of the activated TRN subdivision, echoing its pathway-specific anatomical connections4,6,7. Moreover, these results indicate unique neural mechanism(s) underlying such TRN modulation, whereby it adjusts cortical excitability depending on cross-modal sensory inputs3,5. For example, enhancement of excitatory inputs under the visual only stimulation condition and suppression of inhibitory/competing inputs under the visual-somatosensory competition condition. In summary, our study suggests that modality-specific TRN subdivisions can gate cross-modal sensory processing beyond thalamic level within their corresponding pathways and in an input-dependent manner.Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (R7003-19F, HKU17112120 and HKU17127121 to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), Lam Woo Foundation, Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001) to E.X.W. We would like to thank Jiachen Sun for his technical assistance.References
1. Stein, B.E. & Stanford, T.R. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci 9, 255-266 (2008).
2. Ahrens, S., Jaramillo, S., Yu, K., Ghosh, S., Hwang, G.R., Paik, R., Lai, C., He, M., Huang, Z.J. & Li, B. ErbB4 regulation of a thalamic reticular nucleus circuit for sensory selection. Nat Neurosci 18, 104-111 (2015).
3. Halassa, M.M. & Acsady, L. Thalamic Inhibition: Diverse Sources, Diverse Scales. Trends Neurosci 39, 680-693 (2016).
4. Halassa, M.M., Chen, Z., Wimmer, R.D., Brunetti, P.M., Zhao, S., Zikopoulos, B., Wang, F., Brown, E.N. & Wilson, M.A. State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808-821 (2014).
5. Wimmer, R.D., Schmitt, L.I., Davidson, T.J., Nakajima, M., Deisseroth, K. & Halassa, M.M. Thalamic control of sensory selection in divided attention. Nature 526, 705-709 (2015).
6. Li, Y., Lopez-Huerta, V.G., Adiconis, X., Levandowski, K., Choi, S., Simmons, S.K., Arias-Garcia, M.A., Guo, B., Yao, A.Y., Blosser, T.R., Wimmer, R.D., Aida, T., Atamian, A., Naik, T., Sun, X., Bi, D., Malhotra, D., Hession, C.C., Shema, R., Gomes, M., Li, T., Hwang, E., Krol, A., Kowalczyk, M., Peca, J., Pan, G., Halassa, M.M., Levin, J.Z., Fu, Z. & Feng, G. Distinct subnetworks of the thalamic reticular nucleus. Nature 583, 819-824 (2020).
7. Martinez-Garcia, R.I., Voelcker, B., Zaltsman, J.B., Patrick, S.L., Stevens, T.R., Connors, B.W. & Cruikshank, S.J. Two dynamically distinct circuits drive inhibition in the sensory thalamus. Nature 583, 813-818 (2020).
8. Crabtree, J.W. & Isaac, J.T. New intrathalamic pathways allowing modality-related and cross-modality switching in the dorsal thalamus. J Neurosci 22, 8754-8761 (2002).
9. Yu, X.J., Xu, X.X., He, S. & He, J. Change detection by thalamic reticular neurons. Nat Neurosci 12, 1165-1170 (2009).
10. Kimura, A. Diverse subthreshold cross-modal sensory interactions in the thalamic reticular nucleus: implications for new pathways of cross-modal attentional gating function. Eur J Neurosci 39, 1405-1418 (2014).
11. Kimura, A., Imbe, H., Donishi, T. & Tamai, Y. Axonal projections of single auditory neurons in the thalamic reticular nucleus: implications for tonotopy-related gating function and cross-modal modulation. Eur J Neurosci 26, 3524-3535 (2007).
12. Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
Figures