3025
Unravel the Role of Primary Olfactory Cortices in Olfactory Processing via Optogenetic fMRI
Teng Ma1,2,3, Xunda Wang1,2, Linshan Xie1,2, Pit Shan Chong4, Peng Cao3, Pek-Lan Khong3, Lee Wei Lim4, Ed. X Wu1,2,4, and Alex T. L. Leong1,2
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3Department of Diagnostic Radiology, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China, 4School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
The anterior olfactory nucleus (AON) and the piriform cortex (Pir) are the two primary sensory cortices critical for olfaction. Although it is well documented that both cortices overlap significantly in their functions in olfactory processing, molecular and anatomical tracing studies have indicated otherwise. Consequently, our present understanding of the functions of AON and Pir in olfactory processing at the systems level remains incomplete. In this study, we employed optogenetic fMRI to interrogate the role of AON and Pir in processing olfactory inputs and beyond, and the associated long-range olfactory pathways and their spatiotemporal response properties.
Purpose
Recently, the primary olfactory cortices, namely the anterior olfactory nucleus (AON) and piriform cortex (Pir), have received renewed interest in their functional roles in olfaction and beyond1,2. AON and Pir not only represents early-stage olfactory information processing (i.e., direct inputs from olfactory bulb OB for odor identification and discrimination3,4), but has been shown to be vital for olfactory memory functions and learning through odor representations within local micro-circuits1,5,6 and interactions with the hippocampus1,2,7,8. It is well documented that both AON and Pir overlap significantly in their functions in olfactory processing5,9,10. However, molecular analyses and anatomical tracing works have indicated otherwise11. These studies showed that AON and Pir have numerous unique projections to brain regions that do not overlap, suggesting differences in their odor processing and perception functions. At present, our understanding of the functions of AON and Pir in olfactory processing remains incomplete.In this study, we interrogate the role of AON and Pir in olfactory processing at the systems level using optogenetic stimulation in combination with functional MRI (fMRI). Specifically, we aim to characterize the similarities and/or differences in the functional pathways of AON and Pir for olfactory processing, including their spatiotemporal response properties. Here, we employed optogenetic stimulation based on projection targeting12 (i.e., stimulation of the excitatory afferent inputs from OB at AON and Pir) as opposed to the conventional stimulation of AON or Pir excitatory neurons6,13-15 utilized previously. Doing so would enable specific stimulation of regions in AON and Pir that exclusively receives olfactory inputs.
Methods
Animal preparation and optogenetic stimulation: 3μl of AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to OB (7.5mm anterior to Bregma, +1.7mm medial-lateral right hemisphere, -2.2mm from the surface of dura, Figure 1A) of adult rats (200-250g, male, 6 weeks old, SD strain, n=12). Four weeks after injection, an opaque optical fiber cannula (d=450μm) was implanted at AON and Pir (Figure 1B). Note that only the afferent inputs, not neurons, were transfected in AON and Pir. Blue (473nm) light was presented at 1Hz (10% duty cycle, 40mW/mm2), 5, 10, 20 and 40Hz (30% duty cycle, 40mW/mm2) in a block-design paradigm (Figure 1C).fMRI acquisition and analysis: fMRI data were acquired on 7T Bruker scanner using GE-EPI (FOV=32×32mm2, matrix=64×64, α=56°, TE/TR=20/1000ms, and 20 contiguous slices with 1mm thickness). Data were preprocessed before standard GLM analysis was applied to identify significant BOLD responses (p<0.001; FDR corrected).
Results
Distinct AON and Pir long-range pathways and neural activity propagation characteristics upon low frequency stimulation: Overall, optogenetic stimulation of OB afferent inputs at AON at 1Hz evoked bilateral activations in primary olfactory (AON, Pir, olfactory tubercle Tu, entorhinal cortex Ent, amygdala Amg), limbic (orbitofrontal OFC and cingulate Cg cortices), striatal (nucleus accumbens NAc, caudate putamen CPu) and sensorimotor cortical (somatosensory S1, visual VC, auditory AC, motor MC and insular Ins) regions (Fig 2B, D). However, such brain-wide BOLD activations were absent in subsequent trials. Activations were now localized only in ipsilateral AON without propagation to other downstream targets. Meanwhile, 1Hz stimulation of OB afferent inputs at Pir also activated bilateral primary olfactory, limbic, striatal and sensorimotor regions, albeit with overall weaker and more focal responses than that of AON (Fig 2C, E). Activations in limbic regions and some sensory cortices (i.e., S1, VC and AC) did not diminish significantly in subsequent stimulation trials, while activations in primary olfactory regions were now localized in the ipsilateral hemisphere.Additionally, we observed that BOLD activations in Pir, OFC, Ins, NAc, CPu were bilateral when stimulating AON, while they were only ipsilateral when stimulating Pir upon 5, 10, 20, 40Hz stimulations (Fig 3B, D). Altogether, these findings suggest different neural activity propagation characteristics along long-range olfactory pathways between AON and Pir in olfactory processing.
Discussion & Conclusion
In the present study, we demonstrated that the optogenetic stimulation of OB axonal projection terminals at AON and Pir evoked numerous brain-wide targets including olfactory cortices, regions associated with high-order functions, and sensorimotor cortices, with distinct neural activity propagation characteristics. Upon 1Hz optogenetic stimulation, only the first AON stimulation trial showed robust bilateral brain-wide activations, with subsequent trials only eliciting activations in ipsilateral AON, indicating response adaptation. In contrary, the activations evoked by 1Hz Pir stimulation were relatively weak with no such apparent adaptation. Olfactory adaptation, which is essential for detection of a novel olfactory stimulus after repeated exposure to similar odors3,16, is widely believed to mostly originate from olfactory cortical regions with maintained input from OB17,18. In our study, the observation of a strong and robust response adaptation to long-range neural activitiy propagation upon stimulation of OB afferent inputs at AON indicates a likely predominant role of AON, not Pir, in mediating sensory adaptation in the presence of olfactory inputs. Further, robust bilateral activations found when stimulating AON, not Pir, indicates that AON is also predominant in olfactory processing involving interhemispheric interactions19-21.In summary, we revealed the differences in the spatiotemporal response properties of AON and Pir, respectively, across long-range olfactory pathways, indicating fundamentally distinct roles of both primary olfactory cortices in olfactory processing at the systems level.
Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L., and R7003-19F, HKU17112120 and HKU17127121 to E.X.W.), Lam Woo Foundation, Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001) to E.X.W.References
- Aqrabawi, A.J. & Kim, J.C. Olfactory memory representations are stored in the anterior olfactory nucleus. Nat Commun 11, 1246 (2020).
- Strauch, C. & Manahan-Vaughan, D. Orchestration of Hippocampal Information Encoding by the Piriform Cortex. Cereb Cortex 30, 135-147 (2020).
- Gottfried, J.A. Central mechanisms of odour object perception. Nat Rev Neurosci 11, 628-641 (2010).
- Albrecht, J. & Wiesmann, M. Olfactory Pathways. in Encyclopedia of Neuroscience (eds. Binder, M.D., Hirokawa, N. & Windhorst, U.) 3003-3006 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2009).
- Russo, M.J., Franks, K.M., Oghaz, R., Axel, R. & Siegelbaum, S.A. Synaptic Organization of Anterior Olfactory Nucleus Inputs to Piriform Cortex. J Neurosci 40, 9414-9425 (2020).
- Choy, J.M.C., Suzuki, N., Shima, Y., Budisantoso, T., Nelson, S.B. & Bekkers, J.M. Optogenetic Mapping of Intracortical Circuits Originating from Semilunar Cells in the Piriform Cortex. Cereb Cortex 27, 589-601 (2017).
- Meissner-Bernard, C., Dembitskaya, Y., Venance, L. & Fleischmann, A. Encoding of Odor Fear Memories in the Mouse Olfactory Cortex. Curr Biol 29, 367-380 e364 (2019).
- Aqrabawi, A.J. & Kim, J.C. Hippocampal projections to the anterior olfactory nucleus differentially convey spatiotemporal information during episodic odour memory. Nat Commun 9, 2735 (2018).
- Ennis, M., Puche, A.C., Holy, T. & Shipley, M.T. The Olfactory System. in The Rat Nervous System 761-803 (2015).
- Mori, K. & Sakano, H. Olfactory Circuitry and Behavioral Decisions. Annual Review of Physiology, Vol 83, 231-256 (2021).
- Oh, S.W., et al. A mesoscale connectome of the mouse brain. Nature 508, 207-214 (2014).
- Kim, C.K., Adhikari, A. & Deisseroth, K. Integration of optogenetics with complementary methodologies in systems neuroscience. Nat Rev Neurosci 18, 222-235 (2017).
- Hagiwara, A., Pal, S.K., Sato, T.F., Wienisch, M. & Murthy, V.N. Optophysiological analysis of associational circuits in the olfactory cortex. Front Neural Circuits 6, 18 (2012).
- Giessel, A.J. & Datta, S.R. Olfactory maps, circuits and computations. Curr Opin Neurobiol 24, 120-132 (2014).
- Zhu, P., Tian, Y., Chen, Y., Chen, W., Wang, P., Du, L. & Wu, C. Olfactory Optogenetics: Light Illuminates the Chemical Sensing Mechanisms of Biological Olfactory Systems. Biosensors (Basel) 11(2021).
- Kadohisa, M. & Wilson, D.A. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci U S A 103, 15206-15211 (2006).
- Wilson, D.A. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol 79, 1425-1440 (1998).
- Xia, C.Z., Adjei, S. & Wesson, D.W. Coding of odor stimulus features among secondary olfactory structures. Journal of Neurophysiology 114, 736-745 (2015).
- Illig, K.R. & Eudy, J.D. Contralateral Projections of the Rat Anterior Olfactory Nucleus. Journal of Comparative Neurology 512, 115-123 (2009).
- Yan, Z., Tan, J., Qin, C., Lu, Y., Ding, C. & Luo, M. Precise circuitry links bilaterally symmetric olfactory maps. Neuron 58, 613-624 (2008).
- Imai, T. & Sakano, H. Interhemispheric olfactory circuit and the memory beyond. Neuron 58, 465-467 (2008).
Figures
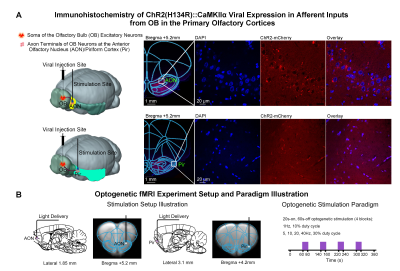
Figure
1. Histological characterization of ChR2 viral expression in the afferent
fibers (i.e., inputs from olfactory bulb OB) in AON & Pir, and optogenetic
setup and stimulation paradigm. (A)
Illustration of optogenetic stimulation based on projection targeting (left),
and confocal images of ChR2 expression in AON (top-right) and Pir (bottom-right).
(B) Illustration of the stimulation site in AON (left) and Pir (middle),
and optogenetic stimulation presented in a block-design paradigm of 20s-on and
60-s off (right).
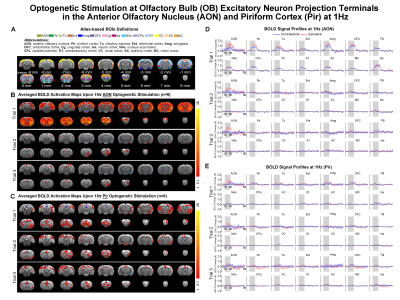
Figure 2. Brain-wide
BOLD activations with subsequent strong response adaptation upon 1Hz
optogenetic stimulation of OB afferent inputs at AON. (A) Atlas defined region of interests (ROIs). (B) Averaged
activation maps of AON stimulation for each of the three successive fMRI trials
(n=6; t>3.1 corresponding to p<0.001). (C) Averaged activation
maps of Pir stimulation (n=6; t>3.1). (D) Corresponding BOLD signal
profiles of AON stimulation extracted from ROIs defined in A. (E) Corresponding
BOLD signal profiles of Pir stimulation. Error bars indicate ± SEM.

Figure 3. Bilateral
vs. ipsilateral only BOLD activations of primary olfactory regions upon 5, 10,
20, 40Hz optogenetic stimulations of OB afferent inputs at AON vs. Pir. (A) Atlas defined ROIs. (B) Averaged
activation maps of AON stimulations (n=6; t>3.1 corresponding to
p<0.001). (C) Averaged activation maps of Pir stimulations (n=6;
t>3.1). (D) Corresponding BOLD signal profiles of AON stimulations extracted
from ROIs defined in A. (E) Corresponding BOLD signal profiles of Pir stimulations.
Error bars indicate ± SEM. (447 characters)
DOI: https://doi.org/10.58530/2022/3025