3023
Radiomics Model to Predict Radiation-induced Temporal Lobe Injury in Nasopharyngeal Carcinoma1Department of Radiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer, Beijing, China, 2National Cancer Center/National Clinical Research Center for Cancer/Cancer, Beijing, China
Synopsis
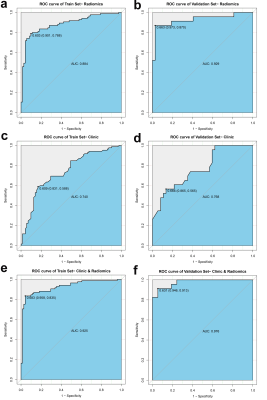
In order to early identify and predict RTLI after IMRT in patients with NPC, we developed and validated a radiomics model based on pretreatment MRI of temporal lobe. The radiomics model demonstrated excellent predictive performance in the validation set (AUC, 0.93; sensitivity, 87%; specificity, 97%). The clinical-radiomics nomogram outperformed clinical nomogram, and showed excellent predictive performance of RTLI in patients within different clinical-pathologic subgroups. These findings suggest that the identified radiomics signature has the potential as a biomarker for risk stratification in RTLI, which may potentially improve the quality of life and prognosis in NPC patients.
Inroduction
The reported rate of radiation-induced temporal lobe injury (RTLI) ranges from 4.6 to 8.5% for patients treated with intensity-modulated radiation therapy (IMRT) radiotherapy1-3. Early intervention has been reported to improve patient prognosis4, the early identification or individualized prediction of RTLI is therefore an important requirement for improving quality of life and prognosis in nasopharyngeal carcinoma (NPC) patients. The purpose of this study is to develop and validate a radiomics-based model for predicting RTLI in NPC by pretreatment Magnetic Resonance Imaging (MRI).Methods
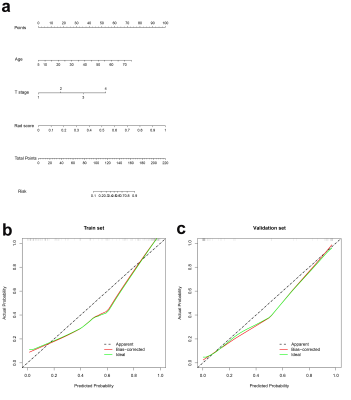
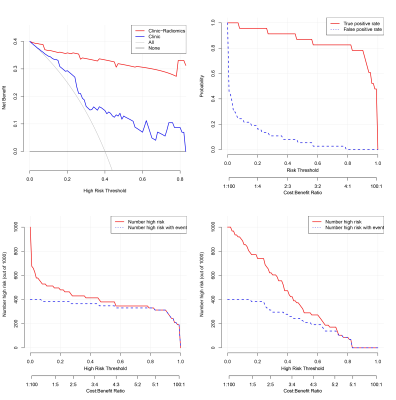
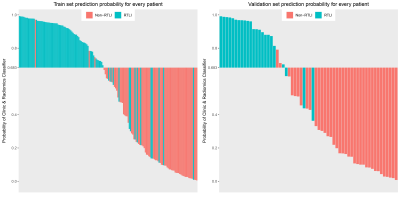
A total of 216 patients with diagnosed NPC between January 2017 and May 2021 were retrospectively reviewed. Patients were randomly allocated to the training (n=156) and the validation cohort (n=60). A total of 1316 radiomics features were extracted from pretreatment contrast-enhanced T1- or T2/fat saturation (FS)-weighted MRI. A radiomics signature was generated by the least absolute shrinkage and selection operator (LASSO) regression algorithm, Pearson correlation analysis, and univariate logistic analysis. Clinical features were selected with logistic regression analysis. Multivariable logistic regression analysis was conducted to develop three models for RTLI prediction in the training cohort: including radiomics signature, clinical variables, and clinical-radiomics parameters, respectively. Further validation of the three models were performed by multivariate logistic regression. Patients were classified into high-risk or low-risk groups according to the clinical-radiomics model, and the threshold was identified by using receiver operating characteristic (ROC) with the area under the curve (AUC) analysis. Stratified analyses were performed by using subgroups within clinical-pathologic factors from the whole data set. A nomogram was drafted integrating the variables included in the best predictive model, which could visualize the weight of different variables in the model. Calibration curves, which indicated the calibration ability of the nomogram, were assessed graphically by plotting the actual observed survival rates and the nomogram-predicted survival rates via a bootstrap method with 1000-iteration resampling. In addition, by calculation of the net benefit for a range of threshold probabilities, decision curve analysis (DCA) was drawn to evaluate the clinical usefulness of the radiomics model.Results
The radiomics signature, composed of three radiomics features, was significantly associated with RTLI. The proposed radiomics model demonstrated favorable differentiation in both the training (AUC, 0.88) and the validation cohort (AUC, 0.93), outperforming clinical prediction model (P<0.05). Combining radiomics and clinical features, higher AUC was achieved (AUC, 0.93 and 0.97 in the training and the validation cohort respectively), as well as a better calibration and improved accuracy of the prediction of RTLI. The DCA showed that the clinical-radiomics model provides a better net benefit to predict RTLI occurrence than the clinical model across the majority of the range of reasonable threshold probabilities. The clinical-radiomics model showed excellent predictive performance of RTLI in patients with different clinical-pathologic subgroups.Discussion
The radiomic features from pretreatment MRI were associated with and could yield accurate prediction of the RTLI occurrence. When the patients were stratified on the basis of clinical-pathologic factors, excellent predictive performances of the radiomics signature were found in all subgroups of clinical-pathologic factors in patients with NPC.Conclusion
A radiomics model derived from pretreatment MRI of the temporal lobe showed persuasive performance for predicting RTLI in NPC.Acknowledgements
Not applicable.References
1. Liang S B, Wang Y, Hu X F, et al. Survival and toxicities of IMRT based on the RTOG protocols in patients with nasopharyngeal carcinoma from the endemic regions of China. J Cancer. 2017,8(18):3718-3724.
2. Su S F, Huang Y, Xiao W W, et al. Clinical and dosimetric characteristics of temporal lobe injury following intensity modulated radiotherapy of nasopharyngeal carcinoma. Radiother Oncol. 2012,104(3):312-316.
3. Zhou G Q, Yu X L, Chen M, et al. Radiation-induced temporal lobe injury for nasopharyngeal carcinoma: a comparison of intensity-modulated radiotherapy and conventional two-dimensional radiotherapy. PLoS One. 2013,8(7):e67488.
4. Chen W, Qiu S, Li J, et al. Diffusion tensor imaging study on radiation-induced brain injury in nasopharyngeal carcinoma during and after radiotherapy. Tumori. 2015,101(5):487-490.
Figures