3017
Disruption on specific segments of uncinate fasciculus in first-episode drug-naïve adolescent depression1Huaxi MR Research Center (HMRRC), Functional and molecular imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital of Sichuan University, Chengdu, China
Synopsis
We aimed to measure diffusion parameters along the uncinate fasciculus and examine the specific and focal alterations of the uncinate fasciculus in first-episode and drug-naïve patients with major depressive disorder in adolescents by using a tract-based quantitative technique. Notably, we found the opposite alterations of the diffusion metrics in the insular and frontal segments of left uncinate fasciculus in adolescents with depression. It will help us to better understand the neurobiological mechanisms of adolescent depression and optimize adolescent-specific treatments for depression.
Background
In adolescent depression, previous neuroimaging studies have revealed the abnormalities in the integrity of the uncinate fasciculus [1-3], a frontolimbic white matter that starts on the lateral nucleus of the amygdala and ends on the medial prefrontal cortex (mPFC) and orbitofrontal cortex (OFC) [4]. However, no study yet has examined the more specific and focal alterations of the uncinate fasciculus in adolescent depression, which may help to provide important insights on the underlying neurobiology and the optimization of treatment for adolescent depression. In the current study, we aimed to measure diffusion parameters along the uncinate fasciculus and examine the focal alterations of the uncinate fasciculus in first-episode and drug-naïve patients with major depressive disorder in adolescents (AMDD) by using a tract-based quantitative technique.Methods
Sixty-one first-episode and drug-naïve AMDD patients diagnosed according DSM-5 criteria and forty-three healthy controls (HC) were recruited from the Third People's Hospital of Mianyang in Sichuan Province, China. All participants were scanned using a 3.0 T Siemens SKYRA MRI scanner with a 20-channel phased-array head coil. The 3D T1-weighted images were acquired with the scanning parameters: TR = 1900 ms; TE = 2.25 ms; data matrix size = 256 × 256; 176 slices; slice thickness =1 mm; flip angle = 9°; voxel size = 1 mm × 1 mm × 1 mm. The diffusion weighted images were measured along 64 noncolinear directions with the scanning parameters: TR = 9200 ms; TE = 95 ms; data matrix size = 128 × 128; 75 slices; slice thickness =2 mm; flip angle = 90°; b value=1000 s/mm2. Diffusion MRI data were processed using FSL (http://www.fmrib.ox.ac.uk/fsl/). The eddy quality control (QC) tool was used to assess the quality of the diffusion-weighted images [5]. There is no motion in any translational directions or rotational directions exceeded 2 mm or 1.5 degrees in each subject. We used the AFQ software package (https://github.com/yeatmanlab/AFQ/) to reconstruct the bilateral uncinate fasciculus. The diffusivity metrics including Fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD) were calculated for 100 equidistant nodes along the fiber tract. According to the previous study [4], the nodes 1-30, 31-72 and 73-100 along the uncinate fasciculus were segmented into temporal, insular and frontal segments, respectively.We used two-sample t-test to compare the four diffusion parameters of each node along the uncinate fasciculus between the AMDD and HC groups. Furthermore, we examined the correlations between HAMD and HAMA and the diffusivity metrics on significant nodes by using Pearson’s correlation. The false discovery rate (FDR) (P<0.05) correction was used for multiple comparisons.
Results
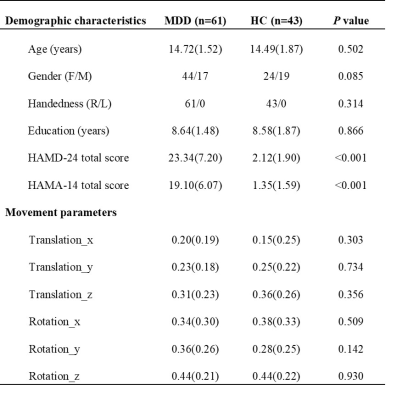
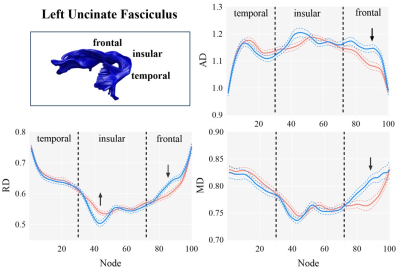
The AMDD and HC groups did not differ significantly in demographic characteristics and motion parameters (Table 1). We used the AFQ software package to generate bilateral uncinate fasciculus for individual participants (Figure 1). For the left uncinate fasciculus, compared with HC group, AMDD group shows significant lower AD, MD and RD on nodes 88-94, 84-93 and 84-91, respectively (FDR-corrected), which is located in the frontal segment of the uncinate fasciculus. While for the insular segment of the uncinate fasciculus, there were higher RD values on nodes 38-44 in AMDD group than HC group (FDR-corrected) (Figure 2). No significant differences were found in the right uncinate fasciculus between the two groups. There were also no significant correlations between HAMD and HAMA and diffusion parameters on significant nodes.Conclusion
To our knowledge, this is the first study to detect opposite alterations of the diffusion metrics in the insular and frontal segments of left uncinate fasciculus, which may explain why previous studies found inconsistent changes of the diffusion metrics in the uncinate fasciculus [1-3]. The uncinate fasciculus is a component of the limbic system that connects temporal and frontal cortex, and is implied associating with emotion regulation [4]. These findings may help us to better understand the neurobiological mechanisms of adolescent depression and optimize adolescent-specific treatments for depression.Acknowledgements
This study was supported by the Science and Technology Project of Sichuan Province (Grant No. 2017JQ0001), the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC21041), and the Clinical and Translational Research Fund of Chinese Academy of Medical Sciences (2021-I2M-C&T-B-097).References
[1] Aghajani M, Veer IM, van Lang ND, et al. Altered white-matter architecture in treatment-naive adolescents with clinical depression. Psychol Med. 2014; 44(11): 2287-2298.
[2] LeWinn KZ, Connolly CG, Wu J, et al. White matter correlates of adolescent depression: structural evidence for frontolimbic disconnectivity. J Am Acad Child Adolesc Psychiatry. 2014;53(8):899-909.e9097.
[3] Ho TC, Sisk LM, Kulla A, et al. Sex differences in myelin content of white matter tracts in adolescents with depression [published online ahead of print, 2021 Jul 2]. Neuropsychopharmacology. 2021;10.1038/s41386-021-01078-3.
[4] Ho TC, King LS, Leong JK, et al. Effects of sensitivity to life stress on uncinate fasciculus segments in early adolescence. Soc Cogn Affect Neurosci. 2017;12(9):1460-1469.
[5] Bastiani M, Cottaar M, Fitzgibbon SP, et al. Automated quality control for within and between studies diffusion MRI data using a non-parametric framework for movement and distortion correction. Neuroimage. 2019;184:801-812.
Figures