3014
Exploring Development Trajectories of White Matter through MR imaging-based Virtual Elastography in Children1the Department of Radiology, the First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Synopsis
Characterizing development trajectories of white matter (WM) is vital for identifying causes of neurodevelopmental disorders. Besides DKI, MRE depicted mechanical properties of brain as a sensitive technique. But WM development trajectories of children were lack of researches on MRE. Therefore, we aimed to investigate age-related development of WM based on virtual elastography, comparing with DKI. We found virtual shear stiffness of WM was positively correlated with age, particularly presenting rapid-growth period of WM before 4-years old, consistent with changes of MK, FA. Virtual elastography may be potentially a valuable technique for depicting WM development trajectories, complementary to diffusion metrics.
Introduction
Characterizing development trajectories of microstructural white matter (WM) is vital for providing clues to identify the root causes of neurodevelopmental and neuropsychiatric disorders. Currently, MR imaging has become the criterion standard for noninvasive high-resolution brain imaging in the pediatric population. Besides the diffusion tensor image and diffusion kurtosis image (DKI) that provide various metrics to quantitatively track evolution of WM maturation1,2, magnetic resonance elastography (MRE) depicted mechanical properties of the brain as a sensitive medical imaging technique that may increase the potential for early diagnosis in recent years3. However, studies of MRE mainly focused on adults, while pediatric population were lack of researches that may be due to safety considerations of mechanical vibrations to developmental brain. It was noteworthy that Le Bihan et al. proposed a hypothesis of diffusion MR imaging–based virtual elastography to provide information on the degree of tissue without using mechanical vibrations4. But, white matter development trajectories of children were lack of researches on virtual elastography. Therefore, this study aimed to investigate the age-related development of white matter based on virtual elastography, and compare with DKI.Materials and Methods
The Institutional Review Broad of the first author’s affiliation approved this study and written informed consent was obtained from parents of the children.Participants Subjects aged 1 months to 12 years were included and underwent DKI. All the subjects were without abnormalities on conventional MRI. We also recruited adults aged 22 years as reference.
MR Protocols DKI was performed on a 3.0T MRI scanner (Signa HDxt, General Electric Medical System, Milwaukee, WI, USA) with an 8-channel head coil. Parameters: directions, 25; b value, 50, 200, 500, 1000, 1500, 2000, 2500s/mm2; SENSE factor, 2; repetition time=11000 ms; echo time=93.9ms; slice thickness=4mm with 4mm gap; field of view=240×240 mm2; matrix=172×172.
Data and statistical analysis DKI raw data were preprocessed by FMRIB software library (FSL; http://www.fmrib.ox.ac.uk/fsl) and fractional anisotropy (FA) and mean kurtosis (MK) were calculated. Then, images of b-value 200 and 1000 s/mm2 were extracted from DKI raw data using Matlab. Diffusion weighted images of the lower b-value (Slow, b value =200 s/mm2) and those of higher b-value (Shigh, b value =1000 s/mm2) were used to estimate virtual shear stiffness4,5: virtual shear stiffness=a·ln (Slow/Shigh)+b. The scaling (a) and the shift (b) factors were separately set to −9.8 and 14 according to the previous calibration studies4,5. Finally, virtual shear stiffness, FA and MK values of splenium of corpus callosum (SCC), genu of corpus callosum (GCC), and bilateral anterior thalamic radiation (ACR), corticospinal tract (CST), inferior longitudinal fasciculus (ILF), and superior longitudinal fasciculus (SLF) were extracted via custom-designed templates according to different ages. Age-related changes of WM-virtual shear stiffness, MK and FA were explored by locally weighted scatterplot smoothing (LOESS), linear regression and Spearman correlation coefficients (r). P<0.05 was considered a statistically significant difference.
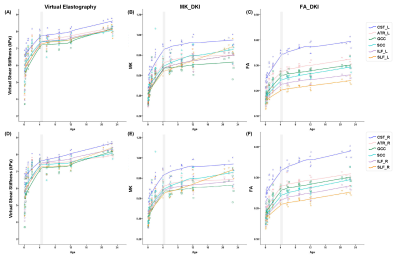
Results
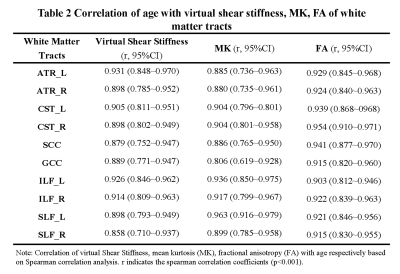
Of Fifty subjects included, 45 were children aged 1 months to 12 years and divided 9 groups with 5 children respectively; the other 5 were adults (Table 1). Significantly postive correlations of virtual shear stiffness, MK, FA with age were observed in above projection, association and commissural fibers (r_vss, range, 0.858~0.931, r_MK, 0.806~0.963, r_FA, 0.806~0.963, P<0.001;) (Table 2). Correlation coefficients of virtual shear stiffness, MK and FA with age were not significantly different. LOESS results showed that age-related changes of the WM-virtual shear stiffness presented a period of rapid growth of WM and slower growth while 4 years old was as the cut-off value, as well as the same age-related tendencies were found in MK and FA (Figure 1). Meanwhile, projection fibers appeared higher virtual shear stiffness, corresponding to higher MK and FA. Particularly, CST appeared highest virtual stiffness among the whole developmental trajectories.Discussion
This study found that WM-virtual shear stiffness was strongly and positively correlated with age, as well as significant age-related changes were observed in MK and FA. With brain development, WM fibers underwent bundle alignment and myelination6, and the virtual shear stiffness was reasonable increased, particularly changed fast at early age. In addition, the LOESS results indicated that WM-virtual shear stiffness proceeds in two phases, with an inflection point at approximately age of 4 years, consistent with known patterns of brain maturation7,8. Additionally, previous study demonstrated that MK may offer a more comprehensive evaluation of age-related microstructural changes1. Our results showed that the same developmental trajectories of WM were observed between virtual stiffness and MK, FA. It may suggest that the virtual shear stiffness could be another complementary metrics to estimate brain maturation. However, currently, our virtual elastography of developing brain was an exploratory research, that the theory was originated from hypothesis in liver4. The relationships of real MRE and virtual elastography in developing brain were needed to further study.Conclusion
Virtual shear stiffness of WM was positively correlated with age, particularly presenting rapid-growth period of WM before 4-years old, consistent with changes of MK, FA. The virtual elastography may be potentially a valuable technique for depicting WM development trajectories, complementary to diffusion metrics.Acknowledgements
This study was supported by National Natural Science Foundation of China (81901516, 82101815, 81901823, 81971581), Shaanxi Provincial Innovation Team (2019TD-018).
* Correspondence: Jian Yang, Ph.D., Professor Department of Radiology The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China E-mail: yj1118@mail.xjtu.edu.cn
References
1. Paydar A, Fieremans E, Nwankwo JI, et al. Diffusional kurtosis imaging of the developing brain. AJNR Am J Neuroradiol. 2014;35(4):808-814.
2. Callaghan MF, Freund P, Draganski B, et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiology of aging. 2014;35(8):1862-1872.
3. Hiscox LV, Johnson CL, Barnhill E, et al. Magnetic resonance elastography (MRE) of the human brain: technique, findings and clinical applications. Physics in medicine and biology. 2016;61(24):R401-r437.
4. Le Bihan D, Ichikawa S, Motosugi U. Diffusion and Intravoxel Incoherent Motion MR Imaging-based Virtual Elastography: A Hypothesis-generating Study in the Liver. Radiology. 2017;285(2):609-619.
5. Lagerstrand K, Gaedes N, Eriksson S, et al. Virtual magnetic resonance elastography has the feasibility to evaluate preoperative pituitary adenoma consistency. Pituitary. 2021;24(4):530-541.
6. Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48-71.
7. Carmody DP, Dunn SM, Boddie-Willis AS, DeMarco JK, Lewis M. A quantitative measure of myelination development in infants, using MR images. Neuroradiology. 2004;46(9):781-786.
8. Parazzini C, Baldoli C, Scotti G, Triulzi F. Terminal zones of myelination: MR evaluation of children aged 20-40 months. AJNR Am J Neuroradiol. 2002;23(10):1669-1673.
Figures