3013
Micro- and macro-structural alterations of white matter following sleep deprivation: a longitudinal fixel-based analysis1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Cardiff University Brain Research Imaging Centre, Cardiff, United Kingdom, 4University of Wisconsin-Madison, Madison, WI, United States
Synopsis
We examined the effect of sleep deprivation (SD) on human brain white matter(WM) using advanced fixel-based analysis. MRI data collected at four morning time points (7-8am, before and after SD, one night recovery and 3 days recovery). Compared to the session before SD, several WM tracts involving in sleep and wakefulness regulation, memory consolidation and emotion response showed varied fiber density (FD), fiber cross-section (FC) and the combined measure of FD and FC (termed FDC) at different time points. These findings demonstrated the heterogeneous sensitivity of WM tracts in response to SD and the microstructural homeostasis.
Introduction
Detrimental effects of sleep deprivation (SD) on human brain white matter have not been fully revealed. A few studies have assessed white matter (WM) integrity after SD using relatively simple modelling techniques which cannot correctly delineate complex WM fiber configurations, leading to misunderstanding and limited biological interpretation[1]. In this study, we examined how the micro- and macro-structural properties of WM are affected by 36 hours SD and recovery sleep longitudinally using advanced fixel-based analysis [2].Methods
Fourteen healthy participants (11 females, aged 24±3.4 years) underwent four MRI scan sessions during 36 hours sustained wakefulness at approximate 12h interval, with the first session started at 7-8 am on the first day, and one MRI scan (at 7am) after 8 hours recovery sleep. Eleven of the 14 participants performed additional MRI scans (at 7am) 3 days later following normal sleep. Then a total of 6 sessions were completed with four morning sessions (S2, S6, S8, S9) and two evening sessions (S3, S7). In each MRI session, high resolution T1 weighted images were acquired using a magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence with the following parameters: TR/TE/TI = 2300/2/857 ms, flip angle = 9°, FOV = 256 mm, voxel size = 1 × 1 × 1 mm3, 192 slices. Multi-shell diffusion MRI (dMRI) data were collected with the following parameters: TR/TE = 3000/59 ms, FOV = 220 mm, voxel size = 2 × 2 × 2mm3, 66 slices, b = 200 s/mm2 (20 directions), b = 500 s/mm2 (20 directions), b = 1200 s/mm2 (30 directions), b = 2400 s/mm2 (60 directions), b = 4000 s/mm2 (60 directions), b = 6000 s/mm2 (60 directions), and 13 interleaved non-diffusion weighted volumes (b = 0) were acquired. An additional reverse phase encoded volume was acquired to correct for magnetic susceptibility-induced distortion during EPI acquisition.dMRI data were processed following the recommended procedures mainly using MRtrix3 toolbox[3], along with FSL and ANTS. The preprocessing steps included: (1) denoising; (2) gibbs ringing removal; (3) eddy, motion and susceptibility induced distortion correction; (4) correction for drift; (5) gradient nonlinearity correction; (6) bias field correction; (7) upsampling dMRI data to isotropic voxel size of 1.25 mm; (8) fiber-orientation distribution (FOD) was estimated in each voxel. An intra-subject template was generated by transforming the FOD maps to their midway space across all sessions for each subject. Then a study specific population FOD template was generated using linear and nonlinear registration of all 14 intra-subject FOD templates. Each subject’s FOD maps were registered to the population template. Fixel-based metrics of fiber density (FD), fiber cross-section (FC) and the combined measure of FD and FC (termed FDC) were calculated. Seventy-two white matter tracts were segmented using TractSeg approach[4], which were converted to fixel maps to segment fixels corresponding to the tracts.
Longitudinal FBA metrics were analyzed across four morning sessions to minimize the effect of circadian rhythm. Fixel-based statistical analysis was performed using connectivity-based fixel enhancement method and non-parametric permutation testing (5000 permutations) with family-wise-error (FWE) corrected p-value for every fixel within the template mask (p < 0.05). Sex and age were regressed out as covariates.
Results
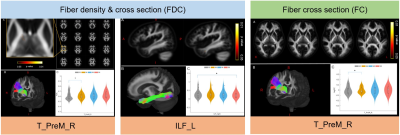
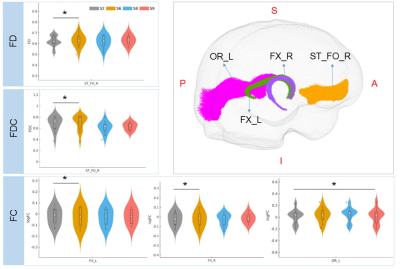
Compared to morning session before sleep deprivation (S2), FDC and FC after 24h SD (S6) significantly decreased in right thalamo-premotor tract, and decreased FDC was observed in left inferior longitudinal fascicle after three days recovery (S9)(Fig 1). In addition, increased FD and FDC in right striato-fronto-orbital tract, increased FC in bilateral fornix after 24h SD, and increased FC in left optic radiation after three days recovery were identified (S9) (Fig 2).Discussion
This longitudinal fixel-based analysis demonstrates that SD may induce regional alterations in axon diameter, extra-axonal space and myelination of whiter matter tracts. The involved tracts play crucial roles in the regulation of states of sleep and wakefulness, memory consolidation and emotion response. Most of the WM changes occur after 24h SD and return back to normal after one night recovery sleep, except that few tracts present sustained variations four days after SD, suggesting that the response timing of white matter tracts to SD is heterogeneous and long-term residual influence of SD exists.Conclusions
SD-induced dynamic micro- and macro-structural alterations of white matter confirms a sleep-dependence white matter homeostasis, which benefits our understanding of the function of sleep on the other hand.Acknowledgements
This study was supported by Wellcome Trust (209192/Z/17/Z); GuangDong Basic and Applied Basic Research Foundation (2021A1515010200); Key Laboratory for Magnetic Resonance and Multimodality Imaging of Guangdong Province (2020B1212060051);References
1. Farquharson, S., et al., White matter fiber tractography: why we need to move beyond DTI. J Neurosurg, 2013. 118(6): p. 1367-77.
2. Dhollander, T., et al., Fixel-based Analysis of Diffusion MRI: Methods, Applications, Challenges and Opportunities. NeuroImage, 2021. 241: p. 118417.
3. Tournier, J.D., et al., MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 2019. 202: p. 116137.
4. Wasserthal, J., P. Neher, and K.H. Maier-Hein, TractSeg - Fast and accurate white matter tract segmentation. NeuroImage, 2018. 183: p. 239-253.
Figures