3010
Lateralization of Memory Function in Temporal Lobe Epilepsy using Scene Memory fMRI1Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Surgery to treat mesial temporal lobe epilepsy (TLE) offers freedom from seizures but risks postsurgical memory decline. Lateralizing memory function is essential for mitigating the risk of resecting “eloquent” cortex. Memory laterality has traditionally been assessed using the Wada test, but it is invasive and poses its own risks. The noninvasive method of functional magnetic resonance imaging (fMRI) has become a possible alternative. However, questions remain about fMRI’s reliability in lateralizing memory. Our group has shown that a scene memory task is capable of activating hippocampus and lateralizing memory function. Here, we present an improved version of that experimental paradigm.
Introduction
Mesial temporal lobe epilepsy (TLE), the most common type of adult onset epilepsy, can be treated with anterior temporal lobe (ATL) resection or laser ablation in medically refractory cases with a 60-80% success rate of curing seizures1,2,3. However, resection or ablation of the ATL, which includes part of the hippocampus, risks postsurgical cognitive decline as the area is critical for multiple aspects of memory4,5.Therefore, presurgical tests for lateralizing memory function are key to improving postsurgical outcome. The gold standard for lateralizing language and memory is the Wada test, but it is highly invasive and its ability to lateralize memory is debatable6. Functional magnetic resonance imaging (fMRI) has emerged as a noninvasive alternative, but evaluating the risk of postoperative memory decline at the individual level remains challenging for a variety of methodological reasons7,8, including difficulty eliciting sufficient task contrast to observe hippocampal activation, which, as part of the default mode network, is active during “rest”.
Prior work from our group has demonstrated that a scene memory fMRI task designed to activate hippocampus in both hemispheres is capable of lateralizing memory function and seizure onset and predicting postsurgical outcome9,10.The protocol has since been improved: First, the original passive baseline task was replaced with an active non-mnemonic task designed to improve task contrast with implicit memory encoding; Second, multiband EPI was used to increase spatial resolution, reducing static susceptibility artifacts in the mesial temporal lobe (mTL); Third, task BOLD data were processed with a state-of-the-art pipeline to reduce physiological noise. In this study, we tested if the improved scene memory task could activate hippocampus and lateralize memory function according to the side of seizure onset. Overall, these results contribute to the body of evidence informing the use of memory fMRI in surgical planning.
Methods
Subjects27 presurgical patients with TLE were analyzed in this study (13 left-sided, 5 right-sided, and 9 bilateral).
fMRI Data Acquisition
All scanning protocols involved a brief localizer scan, high-resolution T1-weighted 3D anatomical imaging (0.8mm isotropic), and gradient-echo echo-planar imaging for BOLD contrast fMRI (2mm isotropic). Imaging was conducted on a 3T Siemens Prisma MRI scanner (Siemens, Germany) using a product T/R head coil.
Functional data preprocessing
The functional data were preprocessed with fMRIPrep 20.0.711. A B0-nonuniformity map was estimated using a phase-difference map and used to correct for susceptibility distortions. The BOLD time-series was resampled to MNI152NLin2009cAsym space. Automatic removal of motion artifacts using independent component analysis12 was performed on the preprocessed BOLD in MNI space after removal of non-steady state volumes and spatial smoothing with an isotropic, Gaussian kernel of 6mm full-width half-maximum.
fMRI Memory Task:
Memory for complex scenes was assessed using a block-design experiment, with seven blocks of novel visual scenes alternating with seven blocks of a control stimulus.
Subjects were instructed to look at the novel scene and judge if the scene was “meaningful” or “not meaningful” to them in some way with a button press. During the active control task, a randomly retiled scene appeared with either the letter “X” or “T” embedded in the image. Subjects pressed a button depending on the letter (Figure 1).
fMRI Data Analysis
Z-score maps of neural activation were generated using FSL FEAT and thresholded at alpha=0.05, FDR-corrected. The Harvard-Oxford atlas was used to define mTL regions (hippocampus, parahippocampal gyrus, fusiform gyrus for each hemisphere). The percentage of suprathreshold voxels among all voxels in each ROI (hippocampus and “mTL”, a composite of all three regions) for each hemisphere was used to calculate a laterality index (LI) for activation using this equation: (left value - right value)/(left value + right value).
Results
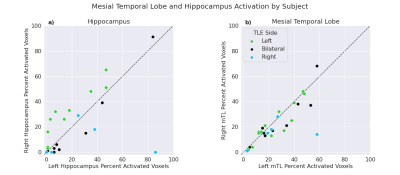
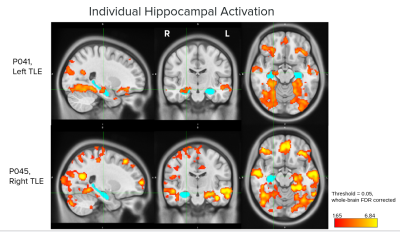
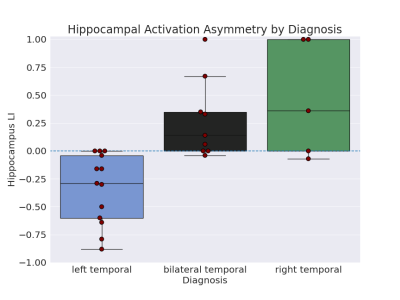
To check if the task was able to elicit hippocampal and mTL activation at an individual level, activation for both left and right hemispheres in each subject was calculated, with 23/27 subjects showing hippocampal activation percentage > 0 in one or both hemispheres and 27/27 in mTL (Figure 2). Examples of hippocampal activity in two subjects are shown in Figure 3.One-tailed t-tests were performed to test if laterality differed significantly from 0 for the left- and right-sided groups. For the left-sided group, mean LI was -0.34 (sd=0.31), significantly different from 0: t(12)=-3.85, p=0.001. For the right-sided group, mean LI was 0.46 (sd=0.49), approaching significance: t(4)=1.97, p=0.06 (Figure 4).
Discussion
These results demonstrate the task is capable of activating hippocampus in most subjects. Four subjects showed no detectable hippocampal activation, which was not attributable to excessive motion or static susceptibility artifact. It may be attributable to poor task compliance, but post-task recognition testing, used in prior work to verify task compliance9,10, was unfortunately not available in this dataset. The task was able to lateralize memory function in the direction predicted by seizure onset, particularly for left-sided patients, potentially because the mostly visuospatial task is biased towards right mTL activity.Conclusion
Although our group has previously found that the task is capable of lateralizing memory function9,10, Towgood, et al. reported that this task was relatively ineffective at lateralization13. However, they used an earlier version of the task and investigated mTL overall, not hippocampus specifically. Here, we show that an improved version reliably activates hippocampus and lateralizes memory function in most subjects.Acknowledgements
R01NS116504References
1. Wiebe, S., Blume, W. T., Girvin, J. P., & Eliasziw, M. (2001). A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. New England Journal of Medicine, 345(5), 311–318. https://doi.org/10.1056/NEJM200108023450501
2. Spencer, S. S., Berg, A. T., Vickrey, B. G., Sperling, M. R., Bazil, C. W., Shinnar, S., Langfitt, J. T., Walczak, T. S., & Pacia, S. V. (2005). Predicting long-term seizure outcome after resective epilepsy surgery: The Multicenter Study. Neurology, 65(6), 912–918. https://doi.org/10.1212/01.wnl.0000176055.45774.71
3. Josephson, C. B., Dykeman, J., Fiest, K. M., Liu, X., Sadler, R. M., Jette, N., & Wiebe, S. (2013).
Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology, 80(18), 1669–1676. https://doi.org/10.1212/WNL.0b013e3182904f82
4. Sherman, E. M. S., Wiebe, S., Fay-McClymont, T. B., Tellez-Zenteno, J., Metcalfe, A., Hernandez-Ronquillo, L., Hader, W. J., & Jetté, N. (2011). Neuropsychological outcomes after epilepsy surgery: Systematic review and pooled estimates. Epilepsia, 52(5), 857–869. https://doi.org/10.1111/j.1528-1167.2011.03022.x
5. Eichenbaum, H., Otto, T., & Cohen, N. J. (1994). Two functional components of the hippocampal memory system. Behavioral and Brain Sciences, 17(3), 449–472. https://doi.org/10.1017/S0140525X00035391
6. Sharan, A., Ooi, Y. C., Langfitt, J., & Sperling, M. R. (2011). Intracarotid amobarbital procedure for epilepsy surgery. Epilepsy & Behavior, 20(2), 209–213. https://doi.org/10.1016/j.yebeh.2010.11.013
7. Buck, S., & Sidhu, M. K. (2020). A Guide to Designing a Memory fMRI Paradigm for Pre-surgical Evaluation in Temporal Lobe Epilepsy. Frontiers in Neurology, 10. https://doi.org/10.3389/fneur.2019.01354
8. Parrish, T. B., Gitelman, D. R., LaBar, K. S., & Mesulam, M.-M. (2000). Impact of signal-to-noise on functional MRI. Magnetic Resonance in Medicine, 44(6), 925–932. https://doi.org/10.1002/1522-2594(200012)44:6<925::AID-MRM14>3.0.CO;2-M
9. Mechanic-Hamilton, D., Korczykowski, M., Yushkevich, P. A., Lawler, K., Pluta, J., Glynn, S., Tracy, J. I., Wolf, R. L., Sperling, M. R., French, J. A., & Detre, J. A. (2009). Hippocampal volumetry and functional MRI of memory in temporal lobe epilepsy. Epilepsy & Behavior, 16(1), 128–138. https://doi.org/10.1016/j.yebeh.2009.07.012
10. Rabin, M. L., Narayan, V. M., Kimberg, D. Y., Casasanto, D. J., Glosser, G., Tracy, J. I., French, J. A., Sperling, M. R., & Detre, J. A. (2004). Functional MRI predicts post-surgical memory following temporal lobectomy. Brain, 127(10), 2286–2298. https://doi.org/10.1093/brain/awh281
11. Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, Kent JD, Goncalves M, DuPre E, Snyder M, Oya H, Ghosh SS, Wright J, Durnez J, Poldrack RA, Gorgolewski KJ. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Meth. 2018; doi:10.1038/s41592-018-0235-4
12. Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015 May 15;112:267–77. doi:10.1016/j.neuroimage.2015.02.064.
13. Towgood, K., Barker, G. J., Caceres, A., Crum, W. R., Elwes, R. D. C., Costafreda, S. G., Mehta, M. A., Morris, R. G., von Oertzen, T. J., & Richardson, M. P. (2015). Bringing memory fMRI to the clinic: Comparison of seven memory fMRI protocols in temporal lobe epilepsy: Comparing Memory fMRI Protocols in TLE. Human Brain Mapping, 36(4), 1595–1608. https://doi.org/10.1002/hbm.22726
Figures