3008
Abnormal dynamic functional connectivity in new-onset, drug-naïve adolescent idiopathic generalized epilepsy1Department of Radiology, Chengdu Medical College, Chengdu, China, 2Department of Radiology, The Second People’s Hospital of Yibin, Yibin, China, 3Department of Radiology, Southwest Medical University, Luzhou, China
Synopsis
Dynamic functional connectivity (dFC) is a newly developed analysis strategies which could provide novel understandings of the pathophysiological mechanisms of neuropsychiatric disease. 45 adolescents diagnosed with IGE and 32 well-matched healthy controls completed this study using dynamic functional connectivity (dFC). The decreased dFC between two seed regions(MCC and ParaHipp) and PCC and IPL were part of default mode network(DMN). The impaired DMN in adolescent IGE patients suggest dFC might be more sensitive to reflect the changes of brain function in the early stage of disease and dFC analysis was a promising avenue to deepen our understanding of this disease.
Introduction
Only few studies investigated the brain morphology abnormalities in adolescent patients with new-onset, drug-naïve idiopathic generalized epilepsy (IGE). Dynamic functional connectivity (dFC) is a newly developed analysis strategies which could provide novel understandings of the pathophysiological mechanisms of neuropsychiatric disease (such as Alzheimer disease and schizophrenia) [1]. In the present study, we sought to explore the changes of the gray matter volume in pediatric cohort of new-onset, drug-naïve IGE. Furthermore, the changed brain regions were used as seed to investigate the dFC with the whole brain.Patients and Methods
45 adolescents diagnosed with IGE (age range: 5-18 years, mean age: 11+3.6, males:females=26:19) and 32 matched healthy controls (age range: 5-18, mean age: 10.7+3.2, males:females=21:11). High resolution 3-dimensional T1-weighted images (3D-T1WI) and the resting-state functional resonance images were acquired on a 3.0 T scanner (Intera Achieva; Philips Medical Systems, Amsterdam, the Netherlands), The imaging parameters of 3D-T1WI using with a spoiled gradient recalled (SPGR) sequence are as follows: 176 axial slices with thickness = 1.7 mm, TR =18 ms, TE = 5 ms, flip angle = 30°, FOV = 220 × 220 cm2, and matrix = 256 × 256. The average scanning time was 7 min. Resting-state resonance functional images using a gradient-echo echo-planar imaging (EPI) sequence with the following parameters: TR = 2000 ms, TE = 30 ms, flip angle = 90°, slices = 30, slice thickness = 5 mm, FOV = 240 × 240 mm2, and matrix = 64 × 64, voxel size = 3.75 × 3.75 × 5 mm3. Each scan lasted 400 s (i.e., 200 volumes). Gray matter volume was investigated by voxel-based morphometry (VBM) analysis using the VBM8 toolbox (www.neuro.uni-jena.de/vbm/download) of SPM8 (www.fil.ion.ucl.ac.uk/spm). Two-sample t-test was used to observe the regional gray matter volume abnormalities between the IGE patients and the healthy controls. We used a common sliding-window approach for each participant to obtain the whole-brain resting-state dFC map of each seed. To assess between-group differences in the mean strength and variance values of the dFC in each seed, we used a general linear model (dependent variable, dFC metrics; independent variable, group), with age and sex as covariates.Results
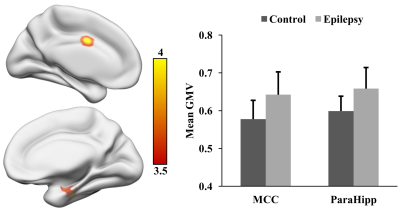
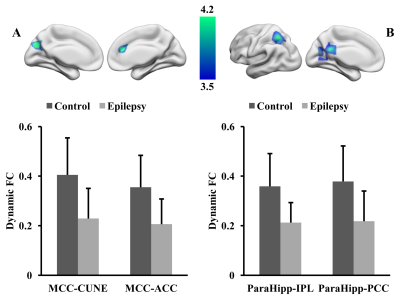
Adolescent IGE patients showed increased gray matter volume in middle cingulate cortex (MCC) and parahippocampus (ParaHipp) as shown in Figure 1. Compared to healthy controls, IGE patients revealed decreased dFC between the middle cingulate cortex and left cuneus (CUNE) and the anterior cingulate cortex (ACC), and decreased dFC between the parahippocampus and posterior cingulate cortex (PCC) and left inferior parietal lobule (IPL) as shown in Figure 2.Discussion
We found increased gray matter volume in middle cingulate cortex and parahippocampus in adolescent IGE patients, and this was different from few previous studies [2, 3] which revealed decreased gray matter volume in IGE patients. These findings might reflect the compensatory adaptation [4] occurred in early stage of disease due to our patients were new-onset, drug-naïve adolescent IGE. The decreased dFC between two seed regions(MCC and ParaHipp) and PCC and IPL were part of default mode network(DMN) [5]. These abnormal dFC suggest that the DMN could be impaired in the early stage of IGE which may be related with the symptoms of unconsciousness and cognitive impairment during absence seizure. The impaired DMN in adolescent IGE patients suggest dFC might be more sensitive to reflect the changes of brain function in the early stage of disease and dFC analysis was a promising avenue to deepen our understanding of this disease.Acknowledgements
We thank all individuals who served as the research participants.References
1. Sakoğlu, U., et al., A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma (New York, N.Y.), 2010. 23(5-6): p. 351-366. 2.
2. Bonilha, L., et al., Voxel-based morphometry reveals gray matter network atrophy in refractory medial temporal lobe epilepsy. Archives of neurology, 2004. 61(9): p. 1379-1384. 3.
3. Kim, E.-H., et al., Structural abnormalities in benign childhood epilepsy with centrotemporal spikes (BCECTS). Seizure, 2015. 27: p. 40-46. 4.
4. Park, J.H., et al., Repeated brief epileptic seizures by pentylenetetrazole cause neurodegeneration and promote neurogenesis in discrete brain regions of freely moving adult rats. Neuroscience, 2006. 140(2): p. 673-684. 5.
5. Greicius, M.D., et al., Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America, 2003. 100(1): p. 253-258.