3002
Functional Imaging of Body Image Distortion In Anorexia Nervosa: Overweight vs. Underweight1Child and Adolescent Psychiatry, Hacettepe University, Ankara, Turkey, 2Psychology, Middle East Technical University, Ankara, Turkey, 3Radiology, Hacettepe University& Bilkent University UMRAM, Ankara, Turkey, 4Bioistatistics, Hacettepe University, Ankara, Turkey, 5Adolescent Health, Hacettepe University, Ankara, Turkey
Synopsis
Body image disorder is the core symptom of Anorexia Nervosa (AN). Our research focuses on a group of adolescent patients diagnosed with AN to investigate neurobiological aspects of body image distortion, comparing the results with healthy control (HC) and major depression (MD) groups. We planned a block-design task in fMRI by using participants' original and distorted overweight and underweight images. We applied General Linear Models (GLM) for whole-brain analyses and examined correlations of the findings with behavioral data. As a result, AN patients differentiated when evaluating their underweight images. However, all adolescents had similar activations for overweight conditions.
INTRODUCTION
Anorexia Nervosa (AN) is a psychiatric disorder typically present in adolescence and characterized by calorie restriction despite very low body weight1. 'Body image distortion' consists of distorted perception, cognition, and emotions related to body weight and shape, which is the core symptom of AN. Although the pathophysiological mechanism of body image disorder is uncertain, aberrant perceptual and cognitive processes may interfere with body image2, 3. However, we have limited knowledge about the 'body image perception' differences between AN and healthy individuals (HC); and other psychiatric disorders that can accompany AN, such as major depression (MD). We designed this cross-sectional case-control study to define the alterations of body image perception in AN using functional magnetic resonance imaging (fMRI).METHOD
Subjects: The sample consisted of 12 adolescent girls diagnosed with AN, nine girls diagnosed with major MD, and ten healthy girls. The mean ages of these groups were 14.8, 15.5, and 14.4 years respectively, and were similar across groups (p>0.05).Procedure: The ethical committee approved the protocol, and all families gave informed consent. Psychiatric diagnostic interview (K-SADS-PL) were applied to all participants to verify the diagnosis. The participants were photographed for task in standard clothes. Their images were modified to obtain overweight (175% size increase at horizontal axis) and underweight (65% size reduction at horizontal axis) images with hidden faces. With 'PsychoPy3 v3.1.5' program, target and fixation blocks (each lasting 16 seconds) were created4. Three blocks consisting of the 'original', 'underweight', 'overweight' images, were randomized and repeated four times. After imaging, participants scored the images for resemblance, satisfaction, and anxiety levels.
Image Acquisition: Imaging was performed on a 3-Tesla MRI scanner (Trio, Siemens). T2-weighted turbo spin-echo (TR = 4000/100 ms; 25 slices; slice thickness 4mm) and T1-weighted high-resolution 3D isovoxel imaging were obtained (MPRAGE;TR/TE:2600/3.1 ms; 176 slices; slice thickness 1mm). The echoplanar-imaging (EPI) sequences applied TR=2000 ms, flip angel=900. Each volume consisted of 28 slices parallel to AC-PC line, (the slice thickness=3.0 mm). The duration of the task was 368 seconds (total duration of imaging:22 minutes) and repeated twice.
Data Processing and Analysis: BrainVoyager QX software package5 was used for pre-processing and whole-brain analysis of fMRI. General linear model(GLM) was performed to analyze group and condition (overweight, underweight and original) interactions. The main effects of groups were analyzed by using single-factor ANOVA. We confirmed the coordinates of the regions using Talairach Daemon Mapping6. For regions with significant main effects, comparisons were performed to detect differences between groups. Correlation analyses (Spearman) were used between the scores given by the participants and β values of regions with significant main effects. Interactions within groups were analyzed with t-tests between three conditions. Bonferroni-Holm correction was applied to determine p value<0.05 at the minimum cluster size. SPSS.21 software was used for behavioral data analysis.
RESULTS
As main effects of groups, occipitotemporal cortex (Broadmann 17,18,19,37) including fusiform gyrus activated for three conditions bilaterally in all groups (all p<0.001). Activations were also seen in bilateral basal ganglia for all groups(all p<0.001). In HC group there were left parahippocampal gyrus and left insula activations (all p<0.001). Interactions within groups are listed in Table 1. We observed wider activations in the occipitotemporal regions for overweight images in all groups compared to other conditions (Figure 1). For occipitotemporal cortex, activation levels between groups in all conditions were similar (all p<0.05). Superior frontal cortex and insula activations in MD and HC groups for underweight images were not seen in AN group. However, there were increased activations in the parietal cortex for underweight images in AN group (Figure 2). We also observed left occipital cortex and left basal ganglia activations for underweight images compared to original images in HC group. When we compared overweight and underweight conditions, we detected activations in occipital cortex and fusiform gyrus bilaterally for all groups. For AN group, participants’ scorings for images were correlated with activations in left caudate nucleus, and right superior temporal cortex. For HC group correlations were found in left insula, and right thalamus.DISCUSSION
In adolescence, worries about appearance are frequently encountered as a developmental feature, independent of psychiatric diagnoses. In addition, in an fMRI study using a similar body image task, both AN patients and healthy individuals demonstrated broad activations for overweight images7. Similarly, activation levels were greater for their overweight images for all participants, as we expected. We conclude that all participants had greater attention to their overweight images. The parietal cortex, insula, and frontal cortex are important areas for visuospatial processing and cognitive and emotional aspects of body image8. In recent studies, the results including these regions are controversial3, 9-16. In our study, both MD and HC groups demonstrated similar activations for underweight images; however, adolescents with AN differed from other adolescents when they evaluated their underweight images.CONCLUSION
In conclusion, our findings may show AN patients' abnormal cognitive and emotional evaluation processes of body image. The brain regions related to these functions, such as the parietal cortex, insula, and frontal cortex, can be essential to investigate further.Acknowledgements
This project was supported by TUBITAK (The Scientific and Technological Research Council of Turkey, Project No: 120S197).
References
1. Association AP. Treatment of patients with eating disorders, American Psychiatric Association. The American journal of psychiatry. 2006;163:4.
2. Epstein J, Wiseman C, Sunday S, Klapper F, Alkalay L, Halmi K. Neurocognitive evidence favors “top down” over “bottom up” mechanisms in the pathogenesis of body size distortions in anorexia nervosa. Eating and Weight Disorders-Studies on Anorexia, Bulimia and Obesity. 2001;6:140-147.
3. Esposito R, Cieri F, di Giannantonio M, Tartaro A. The role of body image and self‐perception in anorexia nervosa: the neuroimaging perspective. Journal of neuropsychology. 2018;12:41-52.
4. Peirce J, Gray J, Halchenko Y, Britton D, Rokem A, Strangman G. PsychoPy–A Psychology Software in Python. 2011.
5. Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single‐subject to cortically aligned group general linear model analysis and self‐organizing group independent component analysis. Human brain mapping. 2006;27:392-401.
6. Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120-131.
7. Castellini G, Polito C, Bolognesi E, D'Argenio A, Ginestroni A, Mascalchi M, Pellicano G, Mazzoni LN, Rotella F, Faravelli C, Pupi A, Ricca V. Looking at my body. Similarities and differences between anorexia nervosa patients and controls in body image visual processing. Eur Psychiatry. 2013;28:427-435.
8. Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110-120.
9. Horndasch S, Roesch J, Kratz O, Vogel A, Heinrich H, Graap H, Moll GH, Dörfler A, Forster C. Neural mechanisms of perceptive and affective processing of body stimuli in Anorexia nervosa–are there developmental effects? Psychiatry research. 2020;286:112853.
10. Miyake Y, Okamoto Y, Onoda K, Kurosaki M, Shirao N, Okamoto Y, Yamawaki S. Brain activation during the perception of distorted body images in eating disorders. Psychiatry Res. 2010;181:183-192.
11. Mohr HM, Zimmermann J, Roder C, Lenz C, Overbeck G, Grabhorn R. Separating two components of body image in anorexia nervosa using fMRI. Psychol Med. 2010;40:1519-1529.
12. Vocks S, Busch M, Grönemeyer D, Schulte D, Herpertz S, Suchan B. Neural correlates of viewing photographs of one's own body and another woman's body in anorexia and bulimia nervosa: an fMRI study. Journal of psychiatry & neuroscience : JPN. 2010;35:163-176.
13. Uher R, Murphy T, Friederich HC, Dalgleish T, Brammer MJ, Giampietro V, Phillips ML, Andrew CM, Ng VW, Williams SC, Campbell IC, Treasure J. Functional neuroanatomy of body shape perception in healthy and eating-disordered women. Biol Psychiatry. 2005;58:990-997.
14. Wagner A, Ruf M, Braus DF, Schmidt MH. Neuronal activity changes and body image distortion in anorexia nervosa. Neuroreport. 2003;14:2193-2197.
15. Via E, Goldberg X, Sánchez I, Forcano L, Harrison BJ, Davey CG, Pujol J, Martínez-Zalacaín I, Fernández-Aranda F, Soriano-Mas C, Cardoner N, Menchón JM. Self and other body perception in anorexia nervosa: The role of posterior DMN nodes. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2018;19:210-224.
16. Sachdev P, Mondraty N, Wen W, Gulliford K. Brains of anorexia nervosa patients process self-images differently from non-self-images: an fMRI study. Neuropsychologia. 2008;46:2161-2168.
Figures
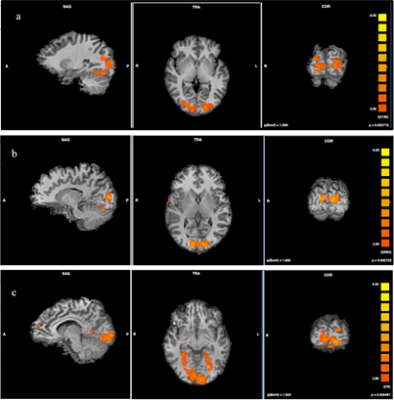
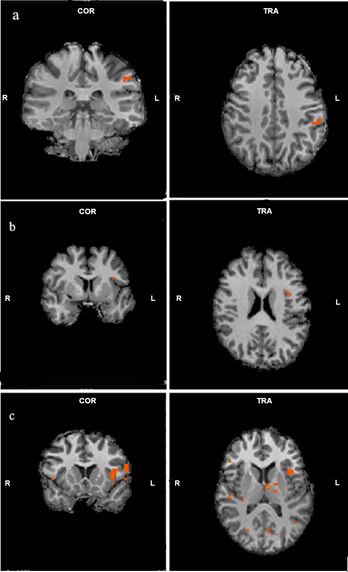

Table 1. Interactions within groups (AN: Anorexia Nervosa, MD: Major Depression, HC: Healthy Controls, L: Left, R:Right)