3001
Using graph convolutional network modeling to characterize functional network disruption in individuals with major depressive disorder1West China Hospital of Sichuan University, Chengdu, China, 2University of Cincinnati College of Medicine, Cincinnati, OH, United States
Synopsis
Few previous studies considered brain network architecture when establishing machine learning models to identify major depressive disorder (MDD). Based on a large, multi-site dataset including 1586 participants, this study aimed to use novel graph convolution network (GCN) to distinguish MDD patients from controls, identify MDD subtypes and characterize related network disruption. We found that GCN enabled excellent classification performance of over 80% accuracy. Besides, shared and distinct disrupted network patterns were identified in first-episode drug-naive and recurrent patients. These findings support the feasibility and effectiveness of network-based GCN classier, illustrating the utility of GCN for detecting disrupted network topology.
Introduction
Increasing studies are dedicated to building accurate machine learning models to characterize major depressive disorder (MDD) and related diagnostic biomarkers at individual level by using neuroimaging data. However, most previous models were based on small sample size, leading to varied performance and inconsistent biological findings. Moreover, such models usually took localized regional brain features as input, ignoring the essence of brain network disruption that has been involved in the pathophysiology of MDD1. Advanced graph convolution network (GCN), as a deep learning technique which allows for direct convolution over graphs, may be one of the optimal model to capture subtle network-level information, and has been proved to achieve excellent performance in Alzheimer's disease and autism2,3. Therefore, the current study aimed to apply graph convolution network (GCN) on large, multi-site MDD dataset of 1586 participants to examine the capability of GCN in characterization of MDD and related brain network disruption at individual level.Methods
Our study was performed based on a large-scale dataset across 16 sites from Rest-meta-MDD consortium that included 821 MDD patients and 765 HC4. Resting-state functional MRI images were acquired from all participants at each local site. A unified image preprocessing protocol was performed using the DPARSF toolbox5. Whole-brain 160×160 functional connectivity matrix was extracted for each individual according to Dosenbach's atlas6. ComBat harmonization were further applied to reduce cross-site heterogeneity which might bias the detection of functional network disruption7.For the input of GCN model, individual whole-brain functional connectivity matrix was first represented as graph structure. Detailed pipeline and architecture of our GCN model is presented in Figure 1. Stratified 10-fold cross-validation was applied to split training and test sets. To identify disrupted brain functional network pattern in MDD, top salient regions contributing to classification were characterized by using class activation map8,9. Post-hoc analysis explored the association between topological features of the identified salient regions (i.e., degree, betweenness and efficiency) and clinical scores. We separately examined the GCN model under different contrasts, including MDD patients vs. controls, first-episode drug-naive (FEDN) patients vs. controls, recurrent patients vs. controls, FEDN vs. recurrent patients.
Results
Under 10-fold stratified cross-validation, GCN achieved accuracy of 81.5% (95%CI: 80.5%-82.5%) for the classification between MDD patients and HC. FEDN patients were distinguished from HC with classification accuracy of 74.1% (95%CI: 72.4%-75.8%), and recurrent patients were distinguished from HC with classification accuracy of 78.1% (95%CI: 76.5%-79.7%). When differentiating between FEDN and recurrent patients, the accuracy were 70.9% (95%CI: 67.7%-74.1%).Based on the GCN model, the top salient regions contributing to group differentiation between MDD patients and HC were primarily located in the DMN, FPN and CON. For the differentiation between FEDN patients and HC, the most salient regions were primarily in the FPN and CON. For the differentiation between recurrent patients and HC, saliency pattern was observed in DMN, FPN and CON, which was similar to the main analysis. The DMN and SMN predominated in the classification between FEDN and recurrent patients.
Among the most salient regions identified via GCN, the left dorsolateral prefrontal cortex (DLPFC) and left IPL exhibited significant association (FDR corrected p < 0.05) with clinical measures. Specifically, the nodal efficiency of the left IPL was negatively associated with depression severity (r = -0.139, p = 0.0002). The nodal degree of the left DLPFC were negatively associated with illness duration (r = -0.133, p = 0.0005) (Figure 3). No significant association between topological characteristics of the salient regions and anxiety severity were observed.
Discussion
This is the first study using large, multi-site dataset to examine the capability of GCN model in identifying MDD patients and characterizing relevant functional network disruptions. The promising performance of GCN model may be ascribed to the consideration of relationship between nodes to capture brain connectome disruption via graphs during model training. The most salient regions contributing to MDD identification were mainly distributed in default mode network (DMN), fronto-parietal (FPN) and cingulo-opercular network (CON). Consistent with previous publications, these networks were related to self-referential processing, cognitive and affective control10, verifying the neurobiological plausibility of the current GCN model. Besides, significant correlation between topological features of salient regions and clinical scores also suggested the capability of GCN in providing clinically-relevant network-level biomarkers.In the subgroup analysis, we observed better classification performance for the identification of recurrent patients compared with that of FEDN patients, indicating more severe brain network dysfunction in patients with long illness course11. Regarding the saliency patterns under different contrasts, since the FPN and CON remained stable across different classification tasks, they may serve as generalizable biomarkers of MDD regardless of illness course and medication status. Besides the DMN was only identified in the recurrent patients, suggesting a secondary salient biomarker besides FPN and CON potentially related to accumulating pathological and medication effects during multiple recurrent depressive episodes12.
Conclusions
These findings based on a large, multi-site dataset support the feasibility and effectiveness of GCN in characterizing individuals with MDD, and also illustrate the potential utility of GCN for detecting clinically-relevant disruption of functional network topology that can enhance understanding of the neurobiology of MDD.Acknowledgements
The authors would like to thank the members of Rest-meta-MDD consortium which provided this multi-site dataset.References
1. Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biological psychiatry. 77, 223-235 (2015).
2. Parisot S, et al. Disease prediction using graph convolutional networks: Application to Autism Spectrum Disorder and Alzheimer's disease. Med Image Anal. 48, 117-130 (2018).
3. Ktena SI, et al. Metric learning with spectral graph convolutions on brain connectivity networks. NeuroImage. 169, 431-442 (2018).
4. Yan CG, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America. 116, 9078-9083 (2019).
5. Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for "Pipeline" Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 4, 13 (2010).
6. Dosenbach NU, et al. Prediction of individual brain maturity using fMRI. Science (New York, NY). 329, 1358-1361 (2010).
7. Yu M, et al. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Human brain mapping. 39, 4213-4227 (2018).
8. Zhou B, Khosla A, Lapedriza A, Oliva A, Torralba A, Ieee. Learning Deep Features for Discriminative Localization. Paper presented at: 2016 Ieee Conference on Computer Vision and Pattern Recognition (CVPR); JUN 27-30, 2016, 2016; Seattle, WA.
9. Arslan S, Ktena SI, Glocker B, Rueckert D. Graph Saliency Maps Through Spectral Convolutional Networks: Application to Sex Classification with Brain Connectivity. Graphs in Biomedical Image Analysis and Integrating Medical Imaging and Non-Imaging Modalities. Second International Workshop, GRAIL 2018 and First International Workshop, Beyond MIC 2018. Held in Conjunction with MICCAI 2018.; 20 Sept. 2018, 2018; Granada, Spain.
10. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA psychiatry. 72, 603-611 (2015).
11. Dohm K, Redlich R, Zwitserlood P, Dannlowski U. Trajectories of major depression disorders: A systematic review of longitudinal neuroimaging findings. The Australian and New Zealand journal of psychiatry. 51, 441-454 (2017).
12. Yang H, et al. Disrupted intrinsic functional brain topology in patients with major depressive disorder. Molecular psychiatry. (2021).
Figures
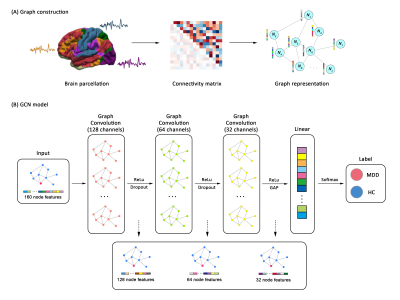
The overall pipeline of graph convolutional network classifier distinguishing between individuals with MDD and HC. (A) Constructing graph structure for each participant using whole-brain resting-state functional connectivity. (B) The architecture and implementation of graph convolutional network.
Abbreviations: ReLu, Rectified Linear Unit; GAP, global average pooling; MDD, major depressive disorder; HC, healthy controls.
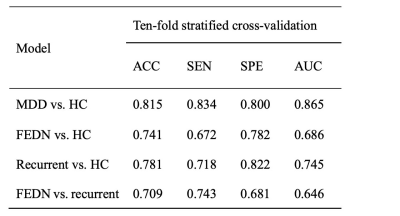
GCN model performance under different classification tasks.
Abbreviations: MDD, major depressive disorder; FEDN, first-episode drug-naïve; HC, healthy control; ACC, accuracy; SEN, specificity; SPE, specificity; AUC, area under receiver operating characteristic curve; GCN, graph convolutional network.
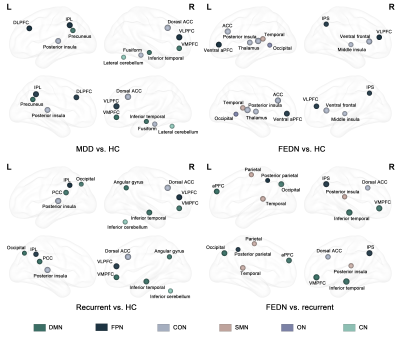
Top 10 salient regions contributing to GCN classification between different groups. Each salient brain region was shown as a sphere in glass brain from lateral and medial view. The size of the sphere reflects the rank of saliency. Region distributed in different networks were shown in different colors.
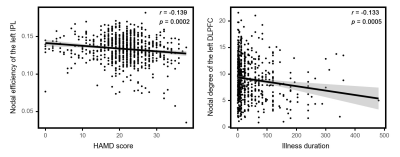
Significant correlation between topological characteristics of the salient regions and clinical measures.The left panel shows the negative correlation between HAMD scores and nodal efficiency of the left IPL, and the right panel shows the negative correlation between illness duration and nodal degree of the left DLPFC. All these correlations survived FDR corrected p < 0.05.