2994
The structure-function coupling of aging brain1Diagnostic Radiology, The University of Hong Kong, Pokfulam, Hong Kong, 2Alzheimer's Disease Research Network, The University of Hong Kong, Pokfulam, Hong Kong, 3The University of Hong Kong, Pokfulam, Hong Kong, 4Rehabilitation Sciences, The Hong Kong Polytechnic University, Hung Hom, Hong Kong
Synopsis
We aim to investigate the effect of aging, sex, years of education, total cognition, and the disease burden of small vessel disease, the most cause of vascular dementia, on not only the coupling of the entire brain, but also the coupling of intra and inter-functional networks. We have demonstrated varying effect of sex, years of education and total cognition on global and intra/inter-network couplings.
Introduction
Static anatomical connections constrain and facilitate the dynamic functional connections between different parts of the brain. One way to jointly characterize the relationship between these two types of brain connections is by the coupling between structural and functional brain networks. 1 In this study, we aim to investigate the effect of aging, sex, years of education, total cognition, and the disease burden of small vessel disease (SVD), the most cause of vascular dementia 2, on not only the coupling of the entire brain, but also the coupling of intra and inter-functional networks 3.Methods
The MRI data (structural, diffusion and resting-state functional MRI) of n = 176 normal subjects (age 62 to 92 years old) were obtained from the Harvard Aging Brain Study 4. These data were collected at two different time points, with 3 years apart. The demographics (age, sex, years of education), and cognitive assessment using the preclinical Alzheimer Cognitive Composite (PACC) 5 were also obtained. The burden of SVD 6 was assessed using the T1, T2, FLAIR and SWI images by a trained scientist, and subsequently verified by a neuroradiologist.All diffusion data were corrected for motion and eddy current geometric distortions using PANDA 7 and FSL. Probabilistic tractography was performed using FSL (probtrackx; number of fibers = 3, weight = 1, burnin = 1000). The brain parcellations by Gordon et al 3 were warped to the individual’s native diffusion space by the inverse transformations of image normalization and coregistration using SPM12. A 333×333 structural connectivity (SC) matrix was subsequently obtained using the fiber counts between different pairs of brain parcellations. The previously described pre and postprocessing procedures 8 of fMRI data was performed using DPARSF 7 and SPM12. Fisher Z-transformation of the pairwise correlation between parcellation-averaged resting-state fMRI activity was performed.
The coupling 1 between structural and functional brain networks was estimated by Spearman-rank correlation between the structural and functional connectomes. The global coupling was estimated from the connectome of the whole brain. The intra-network coupling was estimated from the connections of all the brain regions in the each of the 12 functional networks in Gordon’s parcellation 3, whereas the inter-network coupling was estimated from the connections between the brain regions of one functional network and the brain regions from other functional networks.
The relationships between brain coupling and age, sex, years of education, total cognition and total SVD burden were assessed using linear mixed model (lmer(coupling ~ 1 + age*pacc96 + sex*pacc96 + edu*pacc96 + age*svd + sex*svd + edu*svd + (1|subject)) in R. Since there are altogether 78 different intra and inter-network couplings, the p-values associated with the statistical tests on these coupling were FDR-corrected for multiple comparison.
Results and Discussion
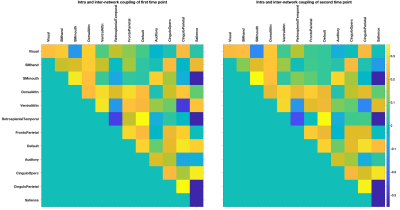
The demographics and clinical variables of n = 176 normal subjects are summarized in Table 1. Figure 1 shows the group-averaged intra and inter-network coupling at two different time points (3 years apart). The statistically significant relationship between coupling versus demographics and clinical variables are summarized in Table 2.The total SVD burden has an interaction effect with years of education only on global coupling (χ2(1) = 5.00, p-value = 0.0254) but not intra and inter-network couplings. Considering the elusive role of SVD burden on cognitive deficit and age-related disability 9 and the protective effect of education on cognition 10, the interaction effect between years of education and total SVD burden on global coupling may indicate that years of education may modulate the effect of SVD on global brain coupling.
Sex affects the intra-network coupling of the somatosensory network of the hand (SMhand; χ2(1) = 12.32, p-value = 0.0254) and inter-network coupling between SMhand and the cingulo-opercular networks (χ2(1) = 12.92, p-value = 0.0148). Years of education (Edu) affects the inter-network coupling between SMhand and auditory networks (χ2(1) = 14.70, p-value = 0.0083). Cognition (PACC; χ2(1) = 18.92, p-value = 9.00e-4) and interaction between cognition and sex (χ2(1) = 20.57, p-value = 0.0004) affect the inter-network coupling between SMhand and dorsal attention (DorsalAttn) networks. The inter-network coupling of DorsalAttn was also found to be associated with cognition in adults between the age of 22 and 37 years old. 11
Table 1
Table 1. Demographics| | Demographics | |
| n | 176 | |
| Sex (F/M) | 111/65 | |
| Years of education | 16.1±3.0 | |
| Number of ApoE4+ | 52 | |
| | | |
| | Time point 1 | Time point 2 |
| Age | 73.1±6.1 | 76.2±6.1 |
| SVD | 1.7±0.8 | 1.8±0.8 |
| PACC96 | 0.11±0.59 | 0.25±0.71 |
Table 2
| Table 2. Effects of demographics and clinical variables on the coupling between structural and functional brain network | ||||||
| | Linear Mixed Model | | Likelihood Ratio Test | |||
| | Estimates | Standard Error | p-value* | | 𝟀2(1) | p-value* |
| Global coupling | | | | | | |
| Edu x SVD | 0.0061 | 0.0028 | 0.0299 | | 5.00 | 0.0254 |
| | | | | | | |
| Intra-network coupling of SMmouth | | | | | ||
| Sex | -0.0933 | 0.0268 | 0.0214 | | 12.32 | 0.0148 |
| | | | | | | |
| Inter-network coupling: | | | | | | |
| SMhand x DorsalAttn | | | | | | |
| PACC | 0.0399 | 0.0092 | 0.0014 | | 18.92 | 9.00e-4 |
| PACC x Sex | -0.0500 | 0.0111 | 0.0006 | | 20.57 | 0.0004 |
| SMhand x Auditory | | | | | | |
| Edu | -0.0479 | 0.0126 | 0.0136 | | 14.70 | 0.0083 |
| SMhand x CinguloOperc | | | | | | |
| Sex | 0.0470 | 0.0132 | 0.0214 | | 12.92 | 0.0148 |
| Edu: Years of education; SVD: total SVD score; SMhand: somatosensory network of the hand; SMmouth: somatosensory network of the tongue; CinguloOperc: cingulo-opercular network; DorsalAttn: dorsal attention network. * after FDR correction | ||||||
Conclusion
In summary, we have demonstrated varying effect of sex, years of education and total cognition on global and intra/inter-network couplings.Acknowledgements
This research is supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region (06172916)References
1. Zhang, Z., Liao, W., Chen, H., Mantini, D., Ding, J. R., Xu, Q., Wang, Z., Yuan, C., Chen, G., Jiao, Q. & Lu, G. Altered functional-structural coupling of large-scale brain networks in idiopathic generalized epilepsy. Brain 134, 2912–2928 (2011).
2. Pinter, D., Enzinger, C. & Fazekas, F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J. Neurol. 262, 2411–2419 (2015).
3. Gordon, E. M., Laumann, T. O., Adeyemo, B., Huckins, J. F., Kelley, W. M. & Petersen, S. E. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb. Cortex 26, 288–303 (2016).
4. Dagley, A., LaPoint, M., Huijbers, W., Hedden, T., McLaren, D. G., Chatwal, J. P., Papp, K. V., Amariglio, R. E., Blacker, D., Rentz, D. M., Johnson, K. A., Sperling, R. A. & Schultz, A. P. Harvard Aging Brain Study: Dataset and accessibility. Neuroimage 144, 255–258 (2017).
5. Donohue, M. C., Sperling, R. A., Salmon, D. P., Rentz, D. M., Raman, R., Thomas, R. G., Weiner, M. & Aisen, P. S. The Preclinical Alzheimer Cognitive Composite. JAMA Neurol. 71, 961 (2014).
6. Wardlaw, J. M., Smith, C. & Dichgans, M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696 (2019).
7. Cui, Z., Zhong, S., Xu, P., He, Y. & Gong, G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 7, (2013).
8. Zhang, H., Chiu, P. W., Ip, I., Liu, T., Wong, G. H. Y., Song, Y. Q., Wong, S. W. H., Herrup, K. & Mak, H. K. F. Small‐World Networks and Their Relationship With Hippocampal Glutamine/Glutamate Concentration in Healthy Adults With Varying Genetic Risk for Alzheimer’s Disease. J. Magn. Reson. Imaging 54, 952 (2021).
9. Pantoni, L., Basile, A. M., Pracucci, G., Asplund, K., Bogousslavsky, J., Chabriat, H., Erkinjuntti, T., Fazekas, F., Ferro, J. M., Hennerici, M., O’Brien, J., Scheltens, P., Visser, M. C., Wahlund, L.-O., Waldemar, G., Wallin, A. & Inzitari, D. Impact of Age-Related Cerebral White Matter Changes on the Transition to Disability – The LADIS Study: Rationale, Design and Methodology. Neuroepidemiology 24, 51–62 (2004).
10. Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., Lindenberger, U., Nyberg, L., Park, D. C., Reuter-Lorenz, P. A., Rugg, M. D., Steffener, J. & Rajah, M. N. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 19, 701–710 (2018).
11. Gu, Z., Jamison, K. W., Sabuncu, M. R. & Kuceyeski, A. Heritability and interindividual variability of regional structure-function coupling. Nat. Commun. 12, 1–12 (2021).