2993
Effect of caffeine on cerebral hemodynamic response: The modulation of dietary caffeine consumption1Department of Biomedical Imaging and Radiological Science, China Medical University, Taichung, Taiwan, China Medical University, Taichung, Taiwan, 2Department of Medical Imaging, China Medical University Hospital, Taichung, Taiwan
Synopsis
The main aim of this study was to characterize the acute effect of caffeine on cerebral hemodynamic responses in participants with different caffeine consumption habits. The non-habitual group exhibited a larger degree of vasoconstriction and thus diminished the ability to dilate upon stimulation. As the vessel dilation ability has been considered as a covariate to explain variabilities in fMRI signals, our results may suggest that the suppressed BOLD response to a visual stimulation in low-caffeine-level users could be partially attributed to the decreased vascular reactivity altered by the baseline perfusion.
Introduction
Caffeine has a significant effect on cerebrovascular systems, and the dual action of caffeine on both neural1 and vascular responses2,3 leads to concerns for the interpretation of blood oxygenation level-dependent (BOLD) functional MRI (fMRI).4 However, potential differences in the brain response to caffeine with regard to the consumption habits have not been fully elucidated, as BOLD responses may vary with the dietary caffeine consumption history. Laurienti et al. proposed that the upregulation of adenosine receptors accompanied by an increase in neural activation may contribute to the enhanced BOLD signal in chronic caffeine users, but not in the non-habitual users.4 Nevertheless, a crucial but less addressed issue is that the BOLD signal depends on not only the neurostimulative effects but also the vasodilation ability.5 Therefore, it is plausible that some of these varied BOLD responses to caffeine could be attributed to vascular alterations. The main aim of this study was to characterize the acute effect of caffeine on cerebral hemodynamic responses including cerebral blood flow (CBF) and cerebrovascular reactivity (CVR) in participants with different patterns of caffeine consumption habits.Methods
Study design: Fifteen participants who were not regular coffee or tea drinkers are referred to as non-habitual group. Eleven participants who consumed more than two cups of coffee per day were referred to as habitual group. The age range was 21 – 29 years old for participants. The study protocol was approved by the local Institutional Review Board.MRI measurement: Magnetic resonance imaging was performed at a 3T scanner (DISCOVERY MR750w, GE, Wisconsin, USA). Each participant was scanned before and after the delivery of 200-mg caffeine ingestion. The MRI protocol consisted of a T1-weighted fast spoiled gradient echo (FSPGR), a pseudo-continuous arterial spin labeling (pCASL) sequence, and BOLD response to breath-hold for CVR measurement. The scan parameters of the FSPGR sequence were as follows: TR/ TE/flip angle=8.02 ms/2.99ms/12°, TI = 450ms, spatial resolution = 1 × 1 × 1 mm3, and number of slices = 170. Scan parameters of the pCASL sequence were as follows: TR/ TE/ flip angle=4600 ms/9.8ms/90°, post labeling delay = 1.8 s, labeling duration = 1.5 s, and 30 pairs of label and control images. The BOLD sequence was implemented with the following parameters: TR/TE/FA = 2000 ms/30 ms/90°, voxel size = 3.5 × 3.5 × 4.4 mm3, and number of slices = 34.
Data analysis: The CBF and CVR data were processed using reported procedures.6,7 A paired t-test was used to determine whether alterations in global gray matter (GM) CBF and global GM BOLD signal changes were significantly different after caffeine administration in each group. To assess differences in CBF and BOLD signal changes after caffeine administration between groups, a Student t-test was performed.
Results
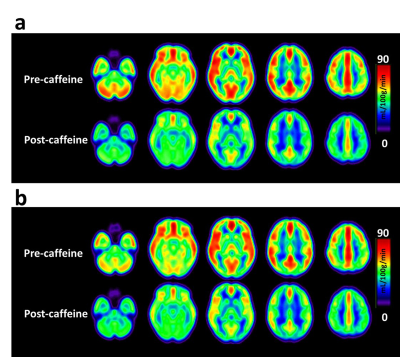
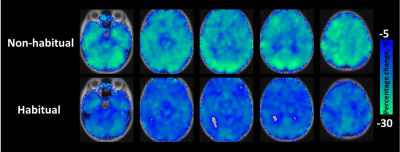
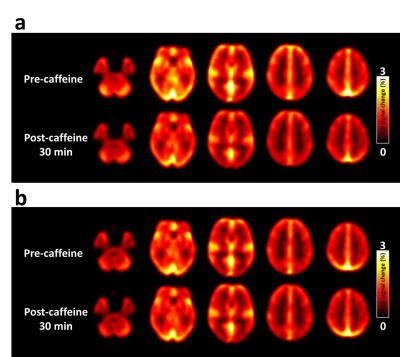
CBF response to caffeine: The absolute CBF maps before and after caffeine administration stratified by group are displayed in Figure 1. The results of the percentage change in cerebral perfusion at 30 min after caffeine administration between the two groups are presented in Figure 2. After 30 min of 200-mg caffeine administration, the global GM CBF were significantly reduced by 21.9% and 12.1% for non-habitual and habitual groups, respectively. The non-habitual group exhibited a larger CBF change than that of the habitual group (P < 0.05).BH-driven responses to caffeine: Figure 3a and 3b show the averaged CVR maps before and after caffeine administration for the non-habitual and habitual groups, respectively. In the non-habitual group, the global GM BOLD signal changes before and after caffeine consumption were 1.3 % ± 0.49 % and 1.03 % ± 0.37 %, respectively. The BOLD response to the BH challenge under the caffeinated condition was significantly lower than that under the baseline condition (P < 0.001). In the habitual group, the global GM BOLD signal changes before and after caffeine consumption were 1.15 % ± 0.35 % and 1.04 % ± 0.32 %, respectively. The levels of BOLD signal changes were not significantly different between the two conditions (P > 0.05), which suggests that the caffeine intervention had an insignificant effect on the CVR measurement in the habitual group.
Discussion and Conclusion
We characterized the acute effects of caffeine on cerebral hemodynamic responses, in participants with different patterns of caffeine consumption habits. The non-habitual group exhibited a larger degree of vasoconstriction and thus diminished the ability to dilate upon stimulation. As the CVR has been considered as a covariate to explain variabilities in fMRI signals,5 our results may suggest that the suppressed BOLD response to a visual stimulation in low-caffeine-level users4 could be partially attributed to the decreased vascular reactivity altered by the baseline perfusion. In the habitual group, the pattern of CBF decrease was smaller, which may exert the minimum influence on the dynamic properties of the vessels. The observations in this study indicate that cerebral hemodynamic responses to caffeine are dependent on caffeine consumption habits. These findings may serve as a potential platform for further studies on BOLD responses to caffeine with the help of CBF and CVR.Acknowledgements
No acknowledgement found.References
1. Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacological reviews 1999; 51(1): 83-133.
2. Xu F, Liu P, Pekar JJ, Lu H. Does acute caffeine ingestion alter brain metabolism in young adults? NeuroImage 2015; 110: 39-47.
3. Merola A, Germuska MA, Warnert EA, Richmond L, Helme D, Khot S et al. Mapping the pharmacological modulation of brain oxygen metabolism: The effects of caffeine on absolute CMRO2 measured using dual calibrated fMRI. NeuroImage 2017; 155: 331-343.
4. Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Dietary caffeine consumption modulates fMRI measures. NeuroImage 2002; 17(2): 751-7.
5. Liu P, Hebrank AC, Rodrigue KM, Kennedy KM, Section J, Park DC et al. Age-related differences in memory-encoding fMRI responses after accounting for decline in vascular reactivity. NeuroImage 2013; 78: 415-25.
6. Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L et al. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic resonance in medicine 2015; 73(1): 102-16.
7. Peng SL, Yang HC, Chen CM, Shih CT. Short- and long-term reproducibility of BOLD signal change induced by breath-holding at 1.5 and 3 T. NMR in biomedicine 2020; 33(3): e4195.
Figures