2992
Functional connectome-based brain features are related to hopelessness in healthy adolescents and young adults1Huaxi MR Research Center (HMRRC), Department of Radiology, West China Hospital of Sichuan Uinversity, Chengdu, China
Synopsis
The neural correlations that characterize hopelessness may help identify brain mechanisms and individuals at risk of depression and suicide. Here, we examined the functional connectivity (FC) patterns of resting state associated with hopelessness in healthy later adolescents and young adults by using CPM. We found that the level of hopelessness was negatively correlated with the FC between the right MTG and the bilateral PoG and PrG, as well as the FC between the right cerebellum VI and the left thalamus. The finding suggested that cortical-cerebellum networks underlying negative future expectation processing characterized hopelessness.
Introduction
Hopelessness is defined as negative expectations for self and the future, and is related to depression and suicidal behavior (Beck A, 1990; Hawkon K 2012). The neural correlations that characterize hopelessness may help identify brain mechanisms and individuals at risk of depression and suicide. Connectome-based predictive model (CPM) (Shen X 2017) provides a useful tool to predict clinical symptoms by uses the functional connection network (Finn ES 2015; LeDoux JE 2020). Here, we examined the functional connectivity (FC) patterns of resting state associated with hopelessness in healthy later adolescents and young adults by using CPM.Methods
We randomly recruited 146 healthy right-handed students aged 18-25 from Sichuan University, and excluded individuals with history of learning disabilities or mental disorders. This study was approved by the Local Research Ethics Committee of West China Hospital of Sichuan University. The 3.0T Siemens-Trio MRI scanner was used for MRI data collection in West China Hospital of Sichuan University. The Chinese version of the Beck Hopelessness Scale (BHS) and the Beck Depression Scale (BDI) are used to assess behavior characteristics (Beck, 1961; Yang, 2015). In this study, the Cronbach's alpha of BHS and BDI are respectively 0.87 and 0.83, showing good internal reliability. Here we calculated the Pearson correlation coefficients between node-by-node time series by using the Brainnetome 273 atlas (Fan) and then transform it to z-scores to obtain the FC matrix. Then we identified the whole-brain FC network relation to hopelessness (threshold p<0.001) with leave-one-out cross validation (LOOCV). According to the CPM method, we conducted polynomial fitting to predict the hopelessness score of the test subject with 1000 iterations of the permutation test, using negative and positive FC networks.Results
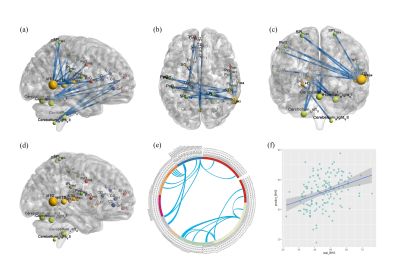
As shown in Figure 1, we found that only the negative network identifying inverse relations between hopelessness and FC predicted the level of hopelessness CPM model (r = 0.3673, corrected p <0.001) but not the positive network (r = 0.0674,uncorrected p = 0.4192). Specifically, the level of hopelessness had a significant negative correlation with the FC in the right MTG with bilateral super parietal lobule (SPL) and postcentral gyrus (PoG), right super temporal gyrus (STG), precentral gyrus (PrG,) and inferior parietal lobule (IPL), and left postcentral gyrus (PoG). Moreover, the negative network relation to hopelessness included the FC between right cerebellum VI and left thalamus, the FC between cerebellum_Vermis_VI and cingulate gyrus.Discussion
In this study, we found that the level of hopelessness was negatively correlated with the FC between the right MTG and the bilateral PoG and PrG, as well as the FC between the right rpSTS and the ipsilateral PrG, left SPL, PoG and PrG. The right MTG and rpSTS are part of the DMN, which plays an important role in the metacognitive processes of inferring or reflecting the current mental state of oneself and others (Frith and Frith, 2003). The PoG and PrG belong to the sensorimotor network (SMN), which participates in the representation of somatic perception and emotional response (Liu, M., et al 2021). DNM-SMN abnormalities have also been found in previous studies in depression (Martino, M.,2016). This may explain why the negative FC between the DMN and the SMN makes adolescents in a state of high hopelessness selectively focus on negative emotional information and use more negative language to describe their feelings about the future. This study found that negative FC in the DMN and SMN sensorimotor network mainly contributed to disrupted top-down regulation on negative future expectations, underscoring the critical role of self-cognition in hopelessness. We also found that FC between the right cerebellum VI and the left thalamus was inversely correlated with levels of hopelessness. Cerebellar-thalamic-cortical circuits play an important role in affective cognition. When the Cerebellar-thalamic-cortical circuits does not function properly, the sensory and cognitive information projected to the cortex is skewed. Previous studies have found that negative emotional information exaggeration is related to thalamic dysfunction in adolescent patients with depression (Stoodley2010). Therefore, disorders of the cerebellar-thalamic-cortical circuit lead to deficiencies in the regulatory function of emotional cognition, and individuals tend to interpret events in a negative way, which leads to negative cognition of future expectations.Conclusion
We demonstrated that cortical-cerebellum networks underlying negative future expectation processing characterized hopelessness and can be used to predict depression levels in healthy later adolescents an young adults. The identified hopelessness networks could be considered potential early intervention targets for adolescent depression. These findings highlight the potential of functional connectivity metrics for understanding neural mechanisms of hopelessness in young individuals who are not clinically depressed.Acknowledgements
Post-Doctor Research Project, West China Hospital, Sichuan University(2020HXBH021)References
1. Beck A, Steer R, Kovacs M, Garrison B. Hopelessness and Eventual Suicide - a 10-Year Prospective-Study of Patients Hospitalized with Suicidal Ideation. American Journal of Psychiatry 1985; 142: 559–63.
2. Hawton K, Saunders KE, O’Connor RC. Self-harm and suicide in adolescents. The Lancet 2012; 379: 2373–82
3. Shen X, Finn ES, Scheinost D, et al. Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols 2017; 12: 506–18.
4. LeDoux JE, Lau H. A new vista in psychiatric treatment: Using individualized functional connectivity to track symptoms. Proceedings of the National Academy of Sciences of the United States of America 2020; 117: 4450–2.
5. Yang, L., Jia, C.-X., & Qin, P. (2015). Reliability and validity of the Center for Epidemiologic Studies Depression Scale (CES-D) among suicide attempters and comparison residents in rural China. BMC Psychiatry, 15(1), 76.
6. Beck, A., Ward, C. H., Mendelson, l., Mock, J., & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571.
7. Frith, U., Frith, C.D., 2003. Development and neurophysiology of mentalizing. Philos.Trans. R. Soc. Biol. Sci. 358, 459–473.
8. Liu, M., Wang, Y., Zhang, A., Yang, C., Liu, P., Wang, J., Zhang, K., Wang, Y., & Sun, N. (2021). Altered dynamic functional connectivity across mood states in bipolar disorder. Brain Research, 1750, 147143.
9. Martino, M., Magioncalda, P., Huang, Z., Conio, B., Piaggio, N., Duncan, N.W., et al., 2016. Contrasting variability patterns in the default mode and sensorimotor networks balance in bipolar depression and mania. Proc. Natl. Acad. Sci. U.S.A. 113 (17), 4824–4829.
10. Stoodley, C. J., & Schmahmann, J. D. (2010). Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 46(7), 831–844