2983
Causal Evidence for a Mouse Homologue of the Human Salience Network via Default Mode Network Inhibition1University of Queensland, Brisbane, Australia
Synopsis
Recent evidence indicates a putative mouse homologue of the human triple-network model, for which a salience network (SN) actively engages either internally-oriented default mode network (DMN) or externally-oriented central executive network (CEN). The interaction amongst these homologues has yet to be validated. This study probed SN responses to DMN inhibition in mice, during functional magnetic resonance imaging. We found that SN radically adjusts its connectivity profile with triple-network components, depending on which DMN hub is inhibited. This indicates that triple-network must be considered holistically, with SN serving an important role in overall triple-network connectivity.
INTRODUCTION
The identification of mouse homologues of human brain networks is important to the development of treatments for human pathology, given the many pathological models available for mice1. The “triple network model”2 describes network relationships amongst an internally-oriented Default Mode Network (DMN) and an externally-oriented Central Executive Network (CEN). Importantly, the Salience Network (SN) is thought to switch the brain between internally- and external-oriented states, by communicating with these two networks. In the human cortex, SN comprises bilateral ventral anterior insula and Anterior Cingulate cortices (ACC)3. Triple-network impairment is implicated in several pathologies, including schizophrenia4 and autism5. Although a mouse DMN is relatively well-established6, a mouse SN is less validated7. A recent study found evidence of a mouse triple-network homologue8, where the function of a putative SN was tested by stimulating the insula directly, prompting altered communication with the remainder of the triple-network. While such a test is important, fully profiling the role of the SN in the triple-network requires perturbation of the other networks, not just the SN. As CEN is not yet well-established for mice, the DMN is a prime candidate for perturbation. The purpose of this study was to use functional magnetic resonance imaging (fMRI), in conjunction with Designer Receptors Exclusively Activated by Designer Drugs (DREADDs), to test the existence of a mouse SN and its functional interactions with a mouse triple-network homologue following DMN deactivation.METHODS
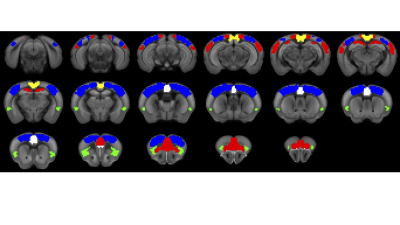
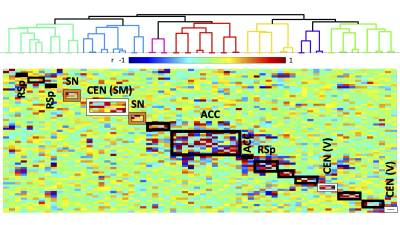
Experiments were approved by the animal ethics committee of the University of Queensland. Four groups of male C57BL/6J mice were used. Two groups underwent surgery for viral vector injection of inhibitory DREADDs in ACC (N=7) and retrosplenial cortex (RSp, N=14), 4.5 weeks prior to fMRI scanning. Two control groups were used: a sham virus control (no DREADDS, N=8), and naïve control (no surgery, N=21). fMRI (TR=0.3s, 0.3x0.3x0.6 mm3 voxels) comprised two 10-minute “resting state” scans, 40 minutes apart, with the second accompanied by intraperitoneal injection of DREADDs agonist Clozapine N-oxide (CNO), to deactivate DMN hubs, with mice lightly anesthetized by isoflurane and medetomidine infusion. Functional connectivity (FC) was measured by partial correlation seed-based connectivity analysis (SCA), using bilateral insula seeds (for SN). Further, 66 seeds were defined based on the SCA results, with 16 in CEN, such as primary motor and somatosensory cortices, and 44 in DMN, such as ACC, RSp and temporal association area (Fig. 1). Hierarchical clustering was performed using FSLNets. FC matrices for baseline and CNO injection data were non-parametrically tested using FSL randomise (p<0.05).RESULTS
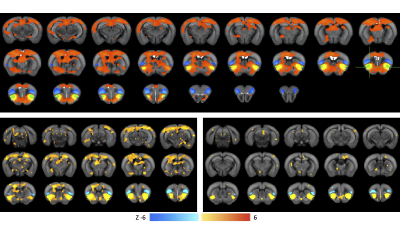
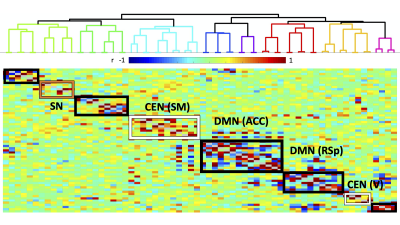
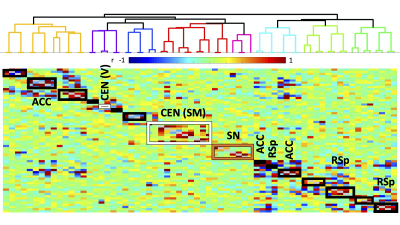
Histology confirmed DREADDS expression in ACC and RSp. SCA using insula seeds indicated a number of visual and somatomotor regions, in addition to DMN regions such as ACC and RSp (p<0.001, FDR correction, Fig 2). For the RSp group, baseline FC clustering indicated that putative SN was more connected to CEN, as compared to DMN, although it also formed a network module with non-medial DMN regions. Further, DMN hubs (ACC/RSp) were quite strongly integrated (Fig. 3). Under RSp inhibition, as expected, the DMN fragmented. Interestingly, SN became more connected with CEN, but was also grouped with parts of the fragmented DMN, including ACC regions (Fig. 4). Non-parametric testing indicated significant insula FC edge decreases, involving insula and CEN regions, or insula and DMN regions except for ACC or RSp. SCA results, post RSp-inhibition, corroborate this finding (Fig 2). Under ACC inhibition, SN fragmented into two lateralized networks, each connected to the CEN (Fig 5). ACC and RSp also became integrated with an overall CEN and SN module. Non-parametric testing indicated significant FC decreases between insula and a few regions of the ACC, however. No significant baseline and CNO differences were found in the sham control groups.DISCUSSION
This is the first study to test functional interactions amongst putative mouse triple-network regions by inhibiting the DMN. SCA results indicate SN communication with a host of regions, including DMN (both RSp and ACC) as well as visual, motor and somatosensory regions. Being externally-oriented processing regions, these latter areas possibly comprise a mouse CEN homologue. This provides further validation of SN communication with triple-network homologues. For the inhibition groups, baseline results indicated that SN communicated more with CEN regions, rather than DMN regions, particularly ACC and RSp. Under RSp inhibition however, SN increased connectivity with CEN and became more integrated with parts of the fragmented DMN. This reconfiguration suggests that the triple-network compensates for a compromised RSp by forming a new FC module amongst CEN, SN and parts of the DMN, possibly to maintain overall balance within the triple-network. This provides a validation for the triple-network hypothesis, since DMN inhibition suffices to alter SN relationships with CEN, not just the DMN itself. Under ACC inhibition, SN fragments into lateralized networks, and the ACC becomes much more integrated with CEN and SN. This intriguing ACC FC change is suggestive of the ACC’s vital role in linking SN and DMN.CONCLUSION
Our results support the contention that a mouse SN homologue both exists and plays an important role in the putative triple-network model for mice. As such, it serves as an important step in the translation of mouse models of neurological and psychiatric diseases, such as schizophrenia and autism.Acknowledgements
No acknowledgement found.References
1. Chuang K, Nasrallah FA. Functional networks and network perturbations in rodents. NeuroImage. 2017. 163:419-436
2. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cog Sci. 2011. 15(10):483-506.
3. Seeley WW. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. JNeurosci. 2019. 39 (50) 9878-9882.
4. Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J Psychiatry Neurosci. 2012. 37(1):17-27.
5. Green S, Hernandez L, Bookheimer SY, et al. Salience Network Connectivity in Autism Is Related to Brain and Behavioral Markers of Sensory Overresponsivity J Am Acad Child Adolesc Psychiatry. 2016. 55(7):618-626
6. Stafford JM, Jarrett BR, Miranda-Dominguez O, et al. Large-scale topology and the default mode network in the mouse connectome. PNAS. 2014 111(52): 18745-18750.
7. Grandjean J, Canella C, Anckaerts C, et al. Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. NeuroImage, 2020, 205:116278.
8. Mandino F, Vrooman RM, Foo HE, et al. A triple-network organization for the mouse brain. Molecular Psychiatry. 2021.
Figures