2982
Establishing causal relationship between resting-state network and behavior1Queensland Brain Institute, The University of Queensland, St Lucia, Australia, 2Centre for Advance Imaging, The University of Queensland, St Lucia, Australia
Synopsis
- Collective influence (CI) analysis provided optimal ranking list of nodes which fastest reduced the giant component of the resting-state networks (RSNs).
- Post-learning inhibition of brain regions which were top CI nodes caused retrograde amnesia in the probe test eight days after spatial learning task.
- Data-driven approaches were used to explore the role of RSNs in memory consolidation.
Introduction
A key question in RSN research is the causal relationship between RSNs, cognitive function, and behaviours. Most studies reported correlation, while only a few studies have examined their causal relationship. Some human studies examined the effects of neuromodulation on altering brain synchronization and behaviors1–3. However, none of them examined the effects of inhibition of RSNs to verify the critical role of specific functional connectivity. With the development of animal fMRI, it has become possible to explore the causal relationship between RSNs and behaviours. There are several studies showing that modulating the network hub of RSNs can significantly affect the RSNs and related behaviors4,5. However, no studies have examined the role of RSNs in high-order functions such as learning and memory. In this study, we identified brain network hubs important for the changed RSNs after a spatial memory task and verified their involvement in memory formation using targeted inhibition.Methods
Experimental designThe study was approved by the animal ethics committee of the University of Queensland. Two experiments were conducted for target selection and validation in C57BL6/J male mice (Fig. 1). For target selection, a group (N=10) underwent active place avoidance (APA), where animals need to learn to avoid a shock zone based on spatial cues, followed by resting-state fMRI scans to find out changed brain networks compared to a control group (N=9), of which no shock was applied. Based on the network analysis, validation was conducted by injecting virus containing inhibitory DREADDs into two identified hubs, LAcbSh (Accumbens nucleus shell, lateral par; N=8) and LO (Lateral orbital cortex; N=8), and compared with another hub with lower-ranking, S1Tr (Primary somatosensory cortex, trunk region, N=8) and two negative control areas, VPM (Ventral posteromedial thalamic nucleus; N=6) and FrA (Frontal association cortex; N=6).
fMRI scan and data pre-processing
Animals went through fMRI scans (9.4T, Bruker) one day and eight days after APA training. Mice were sedated with 0.1 mg/kg/h medetomidine and 0.5-0.25% isoflurane. The resting-state fMRI was acquired using multiband EPI with TR/TE = 300/15 ms, thickness = 0.5 mm, gap = 0.1 mm, 16 axial slices covering the whole cerebrum with in-plane resolution of 0.3 × 0.3 mm2. 2000 volumes were acquired in 10 min and repeated 3 times. Raw data were first pre-processed and then registered to AMBMC mouse brain atlas. The brain was separated into 230 regions and the mean time-series signal of each region was extracted for the construction of correlation matrix.
Target selection
Two-sample t-test was performed between APA and control groups to identify the changed brain networks caused by APA training. CI analysis was performed on the changed networks to create a ranking list according to how fast the giant component can collapse after removing the selected node. Among the top 10 CI nodes, behaviour correlation analysis was applied to select candidate areas for further inhibition experiments (Fig 1A).
Target validation
Inhibitory DREADDs (AAV2/1 pSyn-hM4D(Gi)-T2A-mScarlet) was injected into the target areas under stereotactic surgery. About 4 weeks after surgery, an APA training was conducted. Right after the training, animals were given clozapine N-oxide (CNO, 1mg/kg) via i.p. followed by CNO treatment via drinking water (1mg/kg/day) to keep suppressing the network hub during memory consolidation for seven days. After that, CNO water was changed back to normal water and another session of APA task (probe test) was performed one day later to test memory retention (Fig 1B).
Results
We used four hub centrality algorithms (degree centrality, closeness centrality, betweenness centrality, eigenvector centrality) and one optimal percolation model algorithm (collective influence, CI) to rank the nodes in the network which was obtained by comparing the correlation matrixes of the APA group and control group (p<0.01, uncorrected). The results showed that when the nodes were removed according to the ranking list obtained from CI analysis, the giant component dropped fastest, degree centrality and betweenness centrality the second fast, while closeness and eigenvector centrality were relatively slow (Fig 2). This trend was observed in networks from both post day 1 and post day 8 (Fig 2). These results suggest that the CI analysis provides the optimal ranking list.Fig 3 shows the viral expression behavioural results. After inhibition of LO, LAcbSh, the number of shocks in the probe test was significantly increased compared to their own last training trial (T5) (Figure 3B) while no difference was found in the lower rank hub (S1Tr) and negative control (FrA, VPM) or CNO control groups.
Discussion
Our results showed that inhibiting the key hubs of RSNs during the memory consolidation period can indeed cause retrograde amnesia, demonstrating a causal link between RSNs and behaviours. The lack of behaviour effect by inhibiting lower rank hub indicates that behaviour correlation is not sufficient to provide a causal link. Another highlight of our study is that we used data-driven approaches to explore the role of RSNs in memory consolidation. This is distinguished from previous studies which examined functions of preset networks or brain regions in memory consolidation. Further studies will be required to examine the robustness of the target selection strategy and the mechanisms underlying the involvement of target areas in memory consolidation.Acknowledgements
No acknowledgement found.References
1. Hill, C. A. et al. A causal account of the brain network computations underlying strategic social behavior. (2017). doi:10.1038/nn.4602
2. Violante, I. R. et al. Externally induced frontoparietal synchronization modulates network dynamics and enhances working memory performance. Elife 6, 1–22 (2017).
3. Peña-Gómez, C. et al. Modulation of large-scale brain networks by transcranial direct current stimulation evidenced by resting-state functional MRI. Brain Stimul. 5, 252–263 (2012).
4. Tu, W., Ma, Z. & Zhang, N. Brain network reorganization after targeted attack at a hub region. Neuroimage 237, 118219 (2021).
5. Tu, W., Ma, Z., Ma, Y., Dopfel, D. & Zhang, N. Suppressing Anterior Cingulate Cortex Modulates Default Mode Network and Behavior in Awake Rats. Cereb. Cortex 31, 312–323 (2021).
Figures
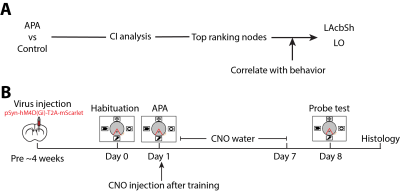
Fig.1 Experimental designs.
(A) Procedure of data processing to identify key hubs. (B) Timeline of DREADDs inhibition experiment.
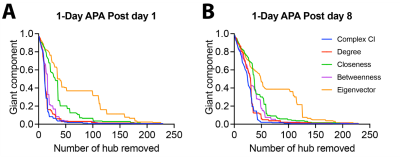
Fig.2 Comparison of five hub selection methods using the giant component index.
Five hub selection methods include CI, degree centrality, closeness centrality, betweenness centrality, and eigenvector centrality. Each line represents the remaining giant component after the nodes (brain regions) are removed from the network one by one according to the rank obtained using each mothed. The quicker the giant component drops, the faster the original network breaks into smaller networks.
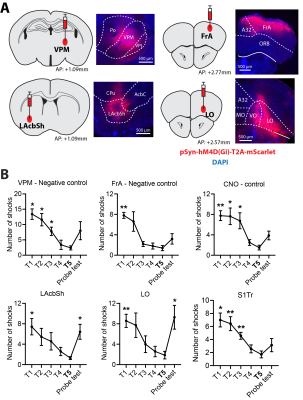
Fig.3 Virus expression and behaviour performance in experimental and control groups.
(A) In each sub-graph, the left image shows the injecting location of the virus and the right image shows the fluorescence imaging. Red fluorescence indicates the location of expression of the virus. (B) The number of shocks in experimental and control groups. All the comparisons are performed between the last training trial (T5) and other training trials or probe tests. * p < 0.05; ** p < 0.01.