2975
Radiomic approach for predicting short-term postoperative recovery of cervical spondylotic myelopathy based on NODDI and T2*WI1Peking University Third Hospital, Beijing, China, 2Engineering Research Center of Bone and Joint Precision Medicine, Beijing, China, 3Beijing Key Laboratory of Spinal Disease Research, Beijing, China, 4MR Collaboration, Siemens Healthineers Ltd, Beijing, China
Synopsis
This study used radiomics based on T2*-weighted imaging (T2*WI) and neurite orientation dispersion and density imaging (NODDI) to predict the short-term recovery of patients with cervical spondylotic myelopathy (CSM). By classifying patients into good and poor outcomes based on the 3-month recovery rate, we found both T2*WI- and NODDI-based radiomic features to have good prognostic power. Furthermore, radiomic score based on NODDI was an independent predictor, whereas the other features were not. These findings suggest that radiomics based on NODDI has good prognostic power for CSM.
Introduction
Cervical spondylotic myelopathy (CSM) is a degenerative disease caused by compression of the spinal cord, which usually results in lifelong disability. Decompression surgery is the main treatment for patients with CSM. However, patients might not benefit from the surgery due to individual differences (1). Physicians attempted to utilize conventional MRI findings such as the grades of increased intensity signal on T2-weighted imaging (T2WI) to predict patients’ post-operative outcomes. Unfortunately, this conventional MRI finding is a subjective visual inspection of lesions in the spinal cord, and its ability for prognosis is limited (2,3).At 3T or higher field strengths, T2*-weighted imaging (T2*WI) provides sharp contrast between spinal cord gray matter and white matter (WM), allowing their segmentation and cross-sectional area measurement. Previous studies revealed that T2*WI is a sensitive method to detect spinal white matter injury and is promising to improve diagnostics and predict outcomes. Neurite orientation dispersion and density imaging (NODDI) is a novel multi-compartment diffusion model based on the assumption of difference of water molecule diffusion in intracellular, extracellular, and cerebrospinal fluid. NODDI characterizes the spinal cord microstructure better and may detect early pathological change in CSM (2,4,5). Radiomics can deeply mine the medical images and provide additional meaningful insights of images (6).
Therefore, this study aimed to find a new prospect for CSM prognosis by using a combination of novel MRI indicators (T2*WI, NODDI) and radiomics approach. We hypothesized that radiomics based on T2*WI and NODDI would be an effective predictive model.
Methods
Sixty-one patients with CSM who underwent preoperative scanning and surgery were retrospectively analyzed. The patients were divided into training (n=46) and testing cohorts (n=15) according to the time series. Patients underwent MRI examinations on a 3T MR scanner (MAGETOM Prisma, Siemens Healthcare, Erlangen, Germany). Parameters for the MRI sequences were Sagittal T2WI TSE: repetition time / echo time=8.5 ms/400 ms; slice thickness=3 mm; scanning time=2:05 min; axial T2* MEDIC: TR/TE=400 ms/17 ms; slice thickness=4 mm; scanning time = 2:31 min; and axial ZOOMit DWI; three shells with b values = 800, 1600, and 2400 s/mm2, each with 64 directions; 5 b0 images (b=0 s/mm2); TR/TE=2000 ms/85 ms; thickness=3 mm; scanning time range=13:04 min.Figure 1 illustrates the procedure for the radiomic analysis, including feature extraction, feature selection, generation of radiomic score (Rad), and construction of a logistic regression model.
Based on patients’ recovery rate at 3-month follow-up, outcomes were divided into good/poor (recovery rate ≥50%/<50%). Clinical features recorded were age, preoperative modified Japanese Orthopaedic Association] score, and symptom duration. Conventional radiological features such as grades of increased signal intensity on sagittal T2WI also were recorded. Clinical and conventional radiological features for later analysis were indicators with P<0.100 in the comparison between good/poor groups on the training cohort, namely Feature_CL and Feature_CR.
Novel MRI-based radiomic features were extracted in the spinal cord at the maximally compressed level from T2*WI and NODDI-derived maps (orientation dispersion index, intracellular volume fraction, and isotropic volume fraction), separately. For T2*WI based radiomic features, the optimal radiomic features were selected through five-fold cross-validation least absolute shrinkage and selection operator (LASSO) from those features with significant difference between good/poor groups on the training cohort. Based on the linear formula in LASSO, Rad1 were generated for T2*WI-based radiomics. In the same way, Rad2, representing NODDI-based radiomics, were computed.
Predictive models were constructed with single factor or multivariable logistic regression based on radiomic features of Rad1, Rad2, Feature_CL and Feature_CR. All logistic regressions were fitted on the training cohort. Their performance on the training and testing cohorts were assessed with receiver operating characteristic (ROC) (Fig 1). Variables with p<0.050 during multivariant analysis were defined as independent factors.
Results
In the training cohort, ages, and increased signal intensity grades were statistically or nearly statistically different between the patients with good/poor outcomes (P=0.005 and 0.070), referred to as Feature_CL and Feature_CR, respectively. For simple logistic analysis, area under the curve (AUC) for models based on Feature_CL, Feature_CR, Rad1, or Rad2 on the training cohort were 0.74±0.07, 0.66±0.07, 0.84±0.06, 0.98±0.02, respectively, and for the testing cohort were 0.79±0.12, 0.54±0.15, 0.70±0.15, and 0.78±0.14, respectively.An individualized prediction model was developed using the multivariable logistic regression analysis and represented by a nomogram (Fig.2). As shown in Fig. 3, the AUC on the training cohort was 0.96±0.02 and on the testing cohort was 0.84±0.13; this result suggests that Rad2 was an independent predictor (coefficient=22.70±11.48, p=0.048), while age, increased signal intensity grades, and Rad1 were not (P=0.356, 0.868, and 0.318, respectively).
Discussion and Conclusion
This is a preliminary study of radiomics based on multi-parameter MRI to predict the early recovery in CSM. The results suggest that conventional radiological and clinical indicators are not sensitive enough to build a predictive model, whereas T2*WI- and NODDI-based radiomic features may be good candidates. Furthermore, radiomics in NODDI is more informative in T2*WI, as Rad2 is an independent predictor while Rad1 is not. The radiomics approach likely will provide more valuable information about the prognosis of CSM, and NODDI-based radiomics has the potential for accurately predicting treatment outcomes of CSM.Acknowledgements
This work was supported by the National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases, Peking University Third Hospital's Research, Innovation and Transformation Fund (BYSYZHKC2020116), Key Clinical Projects of Peking University Third Hospital (BYSY2018003), Beijing Natural Science Foundation (7204327, Z190020), Clinical Medicine Plus X-Young Scholars Project, Peking University, the Fundamental Research Funds for the Central Universities (PKU2021LCXQ005), Capital's Funds for Health Improvement and Research (2020-4-40916), and National Natural Science Foundation of China (82102638) supported this study. We also appreciate the support on the machine learning model and analytical methods from Jing-Jing Cui, Guang-Ming Zhang, and Nan Li.References
1. Iwama T, Ohba T, Okita G, et al. Utility and validity of neurite orientation dispersion and density imaging with diffusion tensor imaging to quantify the severity of cervical spondylotic myelopathy and assess postoperative neurological recovery. Spine J 2020;20:417-425.
2. Zhang MZ, Ou-Yang HQ, Liu JF, et al. Utility of Advanced DWI in the Detection of Spinal Cord Microstructural Alterations and Assessment of Neurologic Function in Cervical Spondylotic Myelopathy Patients. J Magn Reson Imaging 2021;
3. Yagi M, Ninomiya K, Kihara M, Horiuchi Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on Magnetic Resonance imaging. J Neurosurg Spine 2010;12:59-65.
4. Martin AR, De Leener B, Cohen-Adad J, et al. A Novel MRI Biomarker of Spinal Cord White Matter Injury: T2*-Weighted White Matter to Gray Matter Signal Intensity Ratio. AJNR Am J Neuroradiol 2017;38:1266-1273.
5. Martin AR, De Leener B, Cohen-Adad J, et al. Can microstructural MRI detect subclinical tissue injury in subjects with asymptomatic cervical spinal cord compression? A prospective cohort study. BMJ open 2018;8:e019809.
6. Liu Y, Dong D, Zhang L, et al. Radiomics in multiple sclerosis and neuromyelitis optica spectrum disorder. Eur Radiol 2019;29:4670-4677.
Figures
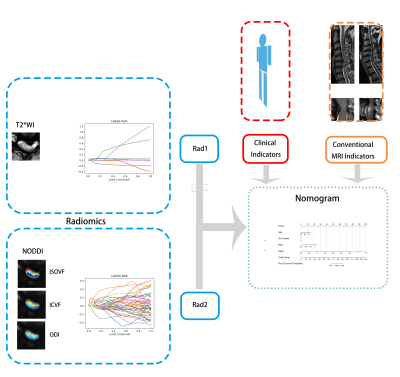
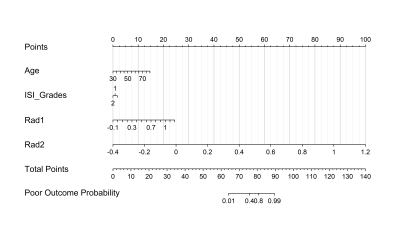
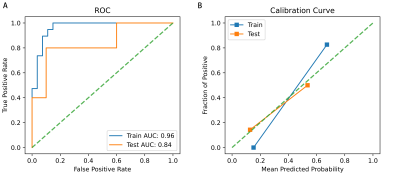
Fig 3. ROCs and calibration curves of the multivariant logistic regression. Blue line (orange line) represents the model’s performance on the training (testing) cohort.
ROC, receiver operating characteristic curve; AUC, area under the curve.