2971
Quantitative assessment of neonatal white matter injury stiffness by virtual magnetic resonance elastography1the first affiliated hospital of Xi'an Jiaotong university, Xi'an, China
Synopsis
Punctate white matter lesion (PWML) is the most common injury in neonates. Due to newborns are a vulnerable population, the limited imaging protocols are not sufficient to fully understand the injury. Le Bihan et.al recently proposed a virtual MR elastography (vMRE) method based on multiple b-values diffusion sequences, which is attractive for evaluation of brain development and injury. This study aims to quantitatively assess the stiffness of PWML using vMRE. Compared with white matter regions, a significant increased virtual sheer stiffness is observed in lesions, and it may be a feasible clinical evaluation of the pathophysiological state of brain tissue.
Introduction
White matter injury is common in neonates, and the most common punctate white matter lesions (PWML) can be detected on conventional MRI as hyper-intensity on T1WI and hypo-intensity on T2WI.[1] Due to the high incidence of PWML (>20%), previous studies have investigated the intensity characteristics, lesion distribution and the white matter microstructural alterations caused by the injury[1, 2]. Up to date, these knowledges are not enough to fully understand this kind of WMI. During the advent of MR elastography, it was a promising approach for describing soft tissue mechanical properties[3], and showed that brain stiffness has great potential to detect biological processes in both health and disease[4]. However, the stiffness of neonatal PWML has not previously been evaluated. Since MR elastography lacks in image resolution, relies on dedicated hardware and software, and adds to examination time, it has limitations in the application of neonatal brain imaging[5]. Recently, Le Bihan et.al proposed a novel method that based on a clinically available diffusion MRI sequences, known as virtual elastography (vMRE), is attractive for evaluation of brain development and injury[5]. Thus, this study aims to quantitatively assess the stiffness of PWML using vMRE.Methods
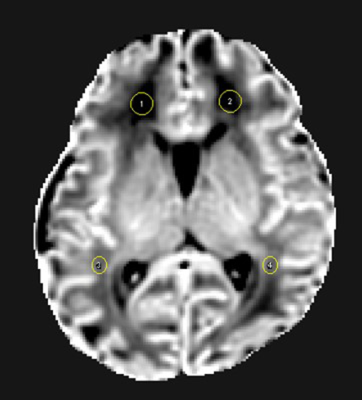
The local institutional review board approved this study and all the written informed consents were obtained from parents of neonates. Subjects Neonates with evidence of PWML (diagnosed by conventional MRI were included. Subjects with obvious imaging artifacts were excluded. MRI Protocols All MR examinations were performed using a 3T scanner (Signa HDxt, GE Healthcare, Milwaukee, Wisconsin) with an 8-channel head coil. The parameters of DKI sequence were as follows: TR/TE=8000-10000ms/91.7-126.1ms; the number of b0=4; b values=0, 50, 200, 500, 1000, 2000, 2500 s/mm2; matrix=128×128; section-thickness=4mm with no gap and FOV=180mm. Data and statistical analysis DKI images of the lower b-value (Slow, b value = 200 s/mm2) and those of the higher b-value (Shigh, b value = 1000 s/mm2) were used to estimate the virtual shear stiffness[5, 6]: virtual shear stiffness = a·ln (Slow/Shigh) + b. The scaling (a) and the shift (b) factors were separately set to −9.8 and 14 according to the previous calibration studies[5, 6]. DKI-derived FA, AK and RK maps were also calculated. Using 3D-T1WI as reference, the manually labeled PWML on virtual shear stiffness maps were also mapped to the DKI parameter maps. The reference ROIs (20±5 mm2) were set in the WM around the anterior and posterior horns of the bilateral lateral ventricles at the basal ganglia level (Figure 1). The values of PWML and WM regions of each subjects were averaged for further analysis. Wilcoxon paired tests were used for the differences DKI parameters and virtual shear stiffness between PWMLs and the matched reference ROIs. All statistical analysis was performed by using SPSS 19.0 (SPSS, Chicago, IL, USA); P<0.05 was considered as statistically significant difference.Results
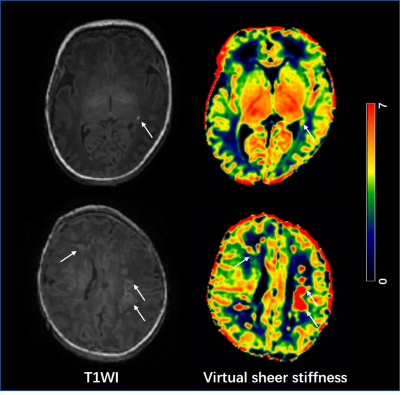
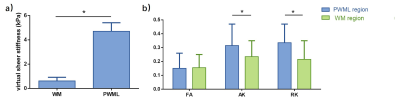
A total of 25 neonates (12 preterm and 13 term subjects, respectively) with PWML were enrolled, and the demographic information is shown in Table 1. Overall, the mean virtual shear stiffness of PWML was significantly higher than that of the surrounding WM regions (P<0.05, Figures 2 and 3a). Compared with reference ROIs, the AK and RK were significantly increased in PWML regions (P<0.001), and no significant difference was observed in FA (P>0.05, Figure 3b).Discussion
This study quantitatively characterized the increased stiffness of neonatal PWML. During the brain development, the myelination, dendritic arborization, synaptogenesis, axonal ramification, glial proliferation and migration and water content may all affect the biomechanical property of brain tissue[7]. In neonatal period, the WM has relatively lower stiffness due to the high water content and incomplete myelination[7]. The increased stiffness of lesions may result from the concentrated of dysmaturity premyelinating oligodendrocytes and reactive activated microglia[8, 9]. Although pesudonormalization of FA may occur in subacute phase of injury, our kurtosis results further confirm the increased complexity or heterogeneity of the microenvironment in lesion regions, which was consistent with previous autopsy studies[10]. Since this method for quantitative evaluation of brain stiffness is an exploratory study based on the diffusion and DKI-based sequences proposed by Le Bihan et.al[5], and its relationship with the real stiffness of MRE needs further exploration.Conclusion
vMRE is a feasible method for characterizing the stiffness alterations of neonatal PWML, and is helpful for clinical evaluation of the pathophysiological state of brain tissue.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 82101815, 81901516, 81971581, 81771810 and 51706178), and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF-CRF-2015-004).References
[1] Li X, Gao J, Wang M, et al. Characterization of extensive microstructural variations associated with punctate white matter lesions in preterm neonates [J]. American Journal of Neuroradiology, 2017, 38(6): 1228-34.
[2] Guo T, Duerden EG, Adams E, et al. Quantitative assessment of white matter injury in preterm neonates: Association with outcomes [J]. Neurology, 2017, 88(7): 614-22.
[3] Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review [J]. Clinical anatomy (New York, NY), 2010, 23(5): 497-511.
[4] Murphy MC, Huston J, 3rd, Ehman RL. MR elastography of the brain and its application in neurological diseases [J]. NeuroImage, 2019, 187(176-83.
[5] Le Bihan D, Ichikawa S, Motosugi U. Diffusion and Intravoxel Incoherent Motion MR Imaging-based Virtual Elastography: A Hypothesis-generating Study in the Liver [J]. Radiology, 2017, 285(2): 609-19.
[6] Lagerstrand K, Gaedes N, Eriksson S, et al. Virtual magnetic resonance elastography has the feasibility to evaluate preoperative pituitary adenoma consistency [J]. Pituitary, 2021, 24(4): 530-41. [7] deCampo D, Hwang M. Characterizing the Neonatal Brain With Ultrasound Elastography [J]. Pediatric neurology, 2018, 86(19-26.
[8] Miller S, Back S. Pathophysiology of Neonatal White Matter Injury [M]. 2017: 1695-703.e4. [9] Rutherford MA, Supramaniam V, Ederies A, et al. Magnetic resonance imaging of white matter diseases of prematurity [J]. Neuroradiology, 2010, 52(6): 505-21.
[10] Niwa T, de Vries LS, Benders MJ, et al. Punctate white matter lesions in infants: new insights using susceptibility-weighted imaging [J]. Neuroradiology, 2011, 53(9): 669-79.
Figures