2970
Structure-function relationships in the human hippocampus: new insights using track-weighted dynamic functional connectivity1The University of Sydney, Sydney, Australia
Synopsis
The hippocampus is a brain structure central to a broad range of cognitive functions including episodic memory. In recent years, we have developed a greater understanding of the structural and functional connectivity of the human hippocampus. Despite these advances, we lack a detailed understanding of structure-function relationships of cortico-hippocampal connectivity. We addressed this gap by combining high-quality data from the Human Connectome Project with cutting-edge fibre-tracking and track-weighted dynamic functional connectivity methods to quantitatively characterise the relationship between anatomical and functional connectivity of the human hippocampus. Our results contribute to ongoing efforts to characterise structure-function relationships of the hippocampus.
Introduction
The hippocampus is a brain structure that is central to a broad range of cognitive functions including episodic memory1. Recent technical and methodological advances have allowed us to conduct increasingly detailed investigations of structural connectivity (SC) and functional connectivity (FC) of the human hippocampus in-vivo using MRI. Recent studies have leveraged these methods to reveal that different portions of the hippocampus have unique patterns of extrinsic SC and FC with different cortical regions2-6. However, SC and FC of the human hippocampus are most often analysed independently, thereby limiting our ability to understand structure-function relationships of cortico-hippocampal connectivity in the human brain.To address this gap, we investigated the relationship between SC and FC of the human hippocampus using track-weighted dynamic functional connectivity (TW-dFC) mapping7. In brief, TW-dFC allowed us to fuse SC and FC data into a quantitative 4D image (i.e., with spatial+temporal information), which was used to characterise structure-function relationships. We combined high quality data from the Human Connectome Project (HCP) with cutting-edge fibre-tracking8 and TW-dFC7 methods to assess whether TW-dFC is effective in identifying functionally-specific regions within the human hippocampus and, if so, to map functional parcels within the hippocampus and identify the cortical networks associated with each parcel.
Methods
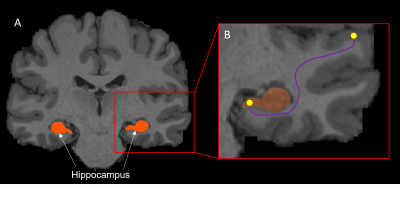
Ten subjects were selected from the HCP 100 unrelated subject database9. Pre-processing of diffusion-weighted images has been described elsewhere10. We first generated 70 million tracks across the entire brain using dynamic seeding. The hippocampus was manually segmented for each participant on the T1-weighted structural image and the segmentation was labelled as ‘5th tissue type’ on a modified-5TT image to allow streamlines to enter the hippocampus rather than terminate at the grey matter/white matter border11,12. Fibre-orientation distribution (FOD) data were used with the modified-5TT image to generate an additional 10 million tracks seeding from the hippocampus. The 70 million whole-brain tracks and 10 million hippocampus tracks were combined and SIFT2 was done on the 80 million track file13. Tracks (and SIFT2 weights) which had an endpoint in the hippocampus were isolated (referred to as the ‘hippocampus tractogram’)12.We calculated the TW-dFC map for the hippocampus tractogram by assigning each streamline a ‘dynamic functional weighting’ given by the functional correlation between resting-state BOLD fMRI data at its end-points7. In essence, this method projects grey matter functional connectivity information to the intersecting white matter pathways (see Figure 1). TW-dFC maps were computed at 2 mm isotropic, corresponding to the native resolution of the fMRI data. The dynamic functional weightings associated with all streamlines traversing a given voxel were averaged to produce the final TW-dFC intensity. The TW-dFC data were further analysed using independent component analysis (ICA; using FSL MELODIC software14). This was carried out at both the single participant and group levels to generate spatial components. The ICA results were used to identify clusters within the hippocampus based on the time-series associated with each hippocampal endpoint in the TW-dFC maps. In essence, this allowed us to investigate, in a data-driven manner, functional clusters in the hippocampus and demonstrate spatial heterogeneity in dynamic functional connectivity within the hippocampus.
Results
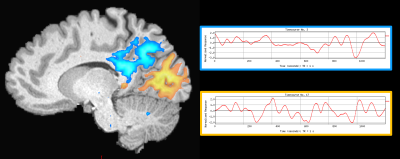
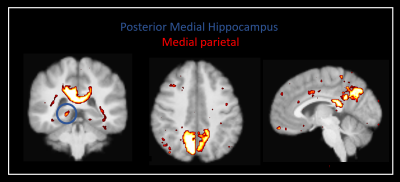
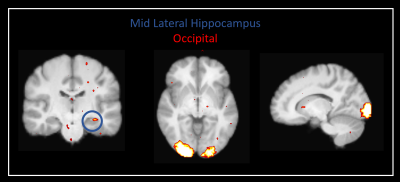
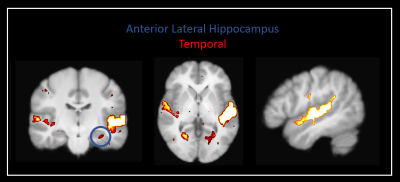
Our method was effective in identifying functionally specific clusters within the human hippocampus. The results of ICA revealed multiple functional clusters along the anterior-posterior axis of the human hippocampus. Each cluster was functionally associated with different cortical areas. For example, at the single participant level, separate clusters in the hippocampus were associated with medial parietal and occipital cortical areas, respectively (see Figure 2A), and each displayed their own dynamic functional fingerprint (given by the time-course of the corresponding spatial ICA component) in relation to the cortical areas they functionally interacted with (see Figure 2B). Group level analysis confirmed that separate clusters within the hippocampus were associated with different cortical networks (see Figures 3-5), each also associate with their own dynamic functional fingerprint.Discussion
Our results revealed how specific regions within the human hippocampus display anatomical and dynamic functional connectivity with distinct cortical areas. We found strong functional associations between the posterior hippocampus and medial parietal regions and, in contrast, between the anterior hippocampus and temporal brain areas. Strikingly, different functional clusters within the hippocampus displayed distinct patterns of cortical connectivity. For example, separate clusters in the hippocampus displayed preferential dynamic functional connectivity with medial parietal, occipital and temporal areas (compare Figures 3-5). Taken together, these observations provide new detailed insights into structure-function relationships within the human hippocampus and have important implications for theories of human hippocampal function along its anterior-posterior axis4,5.Conclusion
Mapping structure-function relationships in the human hippocampus will help us develop more detailed and integrated models of human memory and its biological basis. Overall, our results contribute to ongoing efforts to characterise the relationship between human hippocampal SC and FC with implications for understanding hippocampal function in health and dysfunction in disease.Acknowledgements
Data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University, St. Louis, MO.
We are grateful for the support of the National Health and Medical Research Council of Australia (grant numbers APP1091593 and APP1117724), and the Australian Research Council (grant number DP170101815).
The authors acknowledge the technical assistance provided by the Sydney Informatics Hub and Sydney Imaging, two Core Research Facilities of the University of Sydney, Australia.
References
1. Maguire EA, Mullally SL. The hippocampus: a manifesto for change. J Exp Psychol Gen. 2013;142(4):1180-9.
2. Plachti, S. B. Eickhoff, F. Hoffstaedter, K. R. Patil, A. R. Laird, P. T. Fox, K. Amunts, S. Genon, Multimodal Parcellations and Extensive Behavioral Profiling Tackling the Hippocampus Gradient. Cereb Cortex 29, 4595-4612 (2019).
3. Przezdzik, M. Faber, G. Fernandez, C. F. Beckmann, K. V. Haak, The functional organisation of the hippocampus along its long axis is gradual and predicts recollection. Cortex 119, 324-335 (2019).
4. J. Poppenk, Anatomically guided examination of extrinsic connectivity gradients in the human hippocampus. Cortex 128, 312-317 (2020).
5. B. A. Strange, M. P. Witter, E. S. Lein, E. I. Moser, Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 15, 655-669 (2014).
6. M. A. Dalton, C. McCormick, E. A. Maguire, Differences in functional connectivity along the anterior-posterior axis of human hippocampal subfields. NeuroImage 192, 38-51 (2019).
7. Calamante F, Smith RE, Liang X, Zalesky A, Connelly A. Track-weighted dynamic functional connectivity (TW-dFC): a new method to study time-resolved functional connectivity. Brain Struct Funct. 2017;222(8):3761-3774.
8. Calamante F. The Seven Deadly Sins of Measuring Brain Structural Connectivity Using Diffusion MRI Streamlines Fibre-Tracking. Diagnostics (Basel). 2019;9(3):115.
9. https://db.humanconnectome.org/app/template/SubjectDashboard.vm?project=HCP_1200 &subjectGroupName=100%20Unrelated%20Subjects
10. Civier O, Smith RE, Yeh CH, et al. Is removal of weak connections necessary for graph- theoretical analysis of dense weighted structural connectomes from diffusion MRI? NeuroImage. 2019;194:68-81.
11. Smith RE, Tournier JD, Calamante F, et al. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. NeuroImage. 2012;62:1924–1938.
12. Dalton MA, D’Souza A, Lv J, Calamante F. Anatomical connectivity of the anterior-posterior human hippocampus: new insights using quantitative fibre-tracking. ISMRM & SMRT Annual Meeting 2021.
13. Smith RE, Tournier JD, Calamante F, et al. SIFT2: Enabling dense quantitative assessment of brain white matter connectivity using streamlines tractography. NeuroImage. 2015;119:338-51.
14. Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219.
Figures