2959
Predicting Peritumoral Edema Development after Gamma Knife Radiosurgery of Meningiomas Using Radiomics: A Multicenter Study1Huashan Hospital, Fudan University, Shanghai, China, 2GE Healthcare, Beijing, China, 3Shanghai Gamma Hospital, Huashan Hospital, Fudan University, Shanghai, China
Synopsis
The aim of this study is to adopt machine learning and deep learning methods to predict the risk of post-GKS edema for meningiomas. 595 multicenter cases were included to train and validate 38 random survival forest (RSF) and DeepSurv models. The RSF model incorporating clinical, semantic, and ADC radiomic features achieved the best performance with a C-index of 0.861 in internal validation, and 0.780 in external validation. The derived nomogram had excellent discrimination and calibration. The proposed RSF model with a nomogram represents a non-invasive and cost-effective tool to predict post-GKS edema risks, thus facilitates personalized decision-making in meningioma treatment.
Edema is a complication after Gamma Knife Radiosurgery (GKS) in meningioma patients thatleads to a variety of consequences[1]. The symptoms may necessitate steroid administration and even resection. Potential predictive factors have been investigated but there are so far no easy-to-use tools to predict the risks quantificationally The aim of this study is to construct radiomics-based machine learning (ML) and deep learning (DL) models to predict post-GKS edema development.
Methods
445 meningioma patients who underwent GKS in our institution were enrolled. All participants underwent pre-treatment MRI examinations, including T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and contrast-enhanced T1WI. Apparent diffusion coefficient (ADC) maps were automatically calculated after the DWI acquisition using a mono-exponential fitting method. Data were partitioned into training and internal validation datasets in a ratio of 8:2. 150 cases from multicenter data were also included as the external validation dataset. In each case, 1132 radiomic features were extracted from each pre-treatment MRI sequence (contrast-enhanced T1WI, T2WI, and ADC maps). 9 clinical features and 8 semantic features were also generated. 19 ML (random survival forest, RSF) and 19 DL (DeepSurv) models with different combination of radiomic, clinical, and semantic features were developed with the training dataset, and evaluated with internal and external validation datasets. A nomogram was derived from the model achieving the highest C-index in external validation.
Results
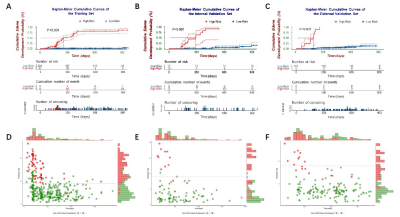
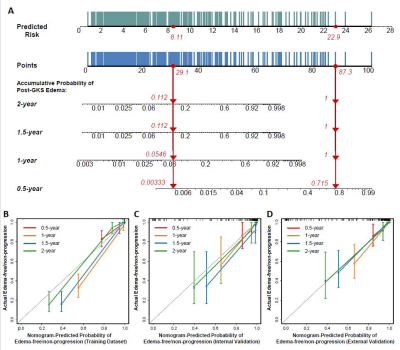
All the models were successfully validated on both validation datasets. The RSF model incorporating clinical, semantic and ADC radiomic features achieved the best performance with a C-index of 0.861 (95% CI: 0.748-0.975) in internal validation, and 0.780 (95% CI: 0.673-0.887) in external validation. It stratifies high-risk and low-risk cases effectively (Figure 1). The nomogram based on the predicted risks provided personalized prediction with a C-index of 0.962 (95%CI: 0.951-0.973) and satisfactory calibration (Figure 2).
Discussion
Previous studies have correlated a variety of risk factors with post-GKS edema in meningioma patients, including a greater therapeutic dose, greater tumor size, non-skull base (particularly parasagittal) location, and presence of pretreatment edema[2]. Our study incorporated radiomic features for the first time which provided abundant quantitative image information that was impossible to obtain with human vision. The radiomic features may predict the post-GKS edema effectively by revealing heterogeneous vasculature aroused by varying VEGF/VPF distribution[3].
Conclusion
Our RSF model with the nomogram represents a non-invasive and cost-effective tool to assist better counselling on the risks, making appropriate treatment decisions individually, and employ customized follow-up plans.
Acknowledgements
No acknowledgement found.References
[1] Hoe Y, Choi YJ, Kim JH, Kwon DH, Kim CJ, Cho YH. Peritumoral brain edema after stereotactic radiosurgery for asymptomatic intracranial meningiomas : Risks and pattern of evolution. J Korean Neurosurg Soc. 2015;58(4):379–84.
[2] Milano MT, Sharma M, Soltys SG, Sahgal A, Usuki KY, Saenz JM, et al. Radiation-Induced Edema After Single-Fraction or Multifraction Stereotactic Radiosurgery for Meningioma: A Critical Review. Int J Radiat Oncol Biol Phy. 2018;101(2):344–57.
[3] Provias J, Claffey K, Lau N, Feldkamp M, Guha A. Meningiomas: Role of Vascular Endothelial Growth Factor/Vascular Permeability Factor in Angiogenesis and Peritumoral Edema. Neurosurgery. 1997;(MAY 1997):1016–26.
Figures