2958
Altered Visual Network and Global Fluctuation in the Resting-state fMRI of Patients with Parkinson’s Disease
Destaw Bayabil Mekbib1, Weiying Dai2, Miao Cai3, Xiaoli Liu3, and Li Zhao1
1Key Laboratory for Biomedical Engineering of Ministry of Education, Zhejiang University, Hangzhou, China, 2Computer Science, Binghamton Univeristy, State University of New York, Binghamton, NY, United States, 3Neurology, Zhejiang Hospital, Hangzhou, China
1Key Laboratory for Biomedical Engineering of Ministry of Education, Zhejiang University, Hangzhou, China, 2Computer Science, Binghamton Univeristy, State University of New York, Binghamton, NY, United States, 3Neurology, Zhejiang Hospital, Hangzhou, China
Synopsis
Resting-state fMRI plays an increasing role in understanding the neural mechanisms of Parkinson's disease (PD). However, neural network alterations, regional and global signal associations are not fully understood in PD patients. Here, we studied the whole-brain neural networks including the inter-network and their correlations with the global fluctuation in 37 PD patients and 20 healthy controls. Our findings revealed that the visual cortex had reduced neural network connectivity in PD patients and altered connections from default mode network to other networks. Weaker correlations were observed between the global signal and regional networks in the PD patients than healthy controls.
Background
Recent studies showed altered brain connectivity in Parkinson’s disease (PD) patients and its potential correlation with alpha-synuclein accumulation 1 using resting-state fMRI studies. For example, malfunctioned default mode network was mainly reported 2. In addition, the interactions among local networks and the contributions of the global fluctuation may provide more information 3. In this work, we investigated the differences in brain connectivity between the patients with PD and healthy controls (HCs) from three perspectives: the voxel-wise resting-state networks (RSNs), the inter-network correlations, and the correlations between global fluctuation and regional networks.Methods
37 PD patients (72.8±11.3, 18 female) and 20 HCs (65.2±13.4, 11 female) were recruited under Institutional Review Boards' permission and scanned using a 3T Siemens Skyra scanner. T1 weighted images were acquired by MPRAGE with an isotropic resolution of 1mm3, TR of 2300ms, TE of 2.3ms, and TI of 900ms. Resting-state blood oxygenation level-dependent (BOLD) fMRI images were acquired with TR of 2s, TE of 30ms, and 33 slices with thickness 3.5 mm and an acceleration factor of 2. 240 volumes were acquired and the first 10 volumes were removed. Images were skull stripped, motion-corrected, spatially smoothed, and temporally filtered. Single-subject independent component analysis (ICA) was used to reduce motion and physiological noises, in which 15 independent components (ICs) were extracted and noise-related ICs were manually removed. Then the images were normalized to the MNI space. First, voxel-wised brain connectivity maps were calculated using group-level ICA. The spatial maps were visually inspected and correlated with a set of reference resting-state networks available in the FSL. Dual regression was conducted to extract subject-specific time courses and associated spatial maps using the group-level spatial maps as regressors 4. A permutation test (5000 permutations) with FSL-randomize was applied on the resulting subject-specific spatial maps to compare differences in voxel-wise RSNs between PD patients and HCs. Second, the inter-network correlation matrix was computed on the combined subject-specific time series. Third, the global signal (GS) time series was calculated as the mean values over the whole brain mask across time and was correlated with the time series of regional networks in the second step. Results were corrected for multiple comparisons by considering family-wise error-corrected (FWE) p-value less than 0.05 as statistically significant. The data were processed using the MELODIC ICA and dual regression of the FSL toolbox (Analysis Group, FMRIB, Oxford, UK.).Results
In the group ICA, 13 of the 15 ICs were considered functionally relevant RSNs including, sensorimotor network (SMN), default mode network (DMN, IC 2 and 3), visual network (VN), central executive network (CEN), cerebellar network (CBN), basal ganglia network (BGN), right frontoparietal network (RFPN), left frontoparietal network (LFPN), frontal cortical network (FCN), right language network (RLN), left language network (LLN), and lateral visual network (LVN), Figure 1. Voxel-wise group differences were conducted on the 13 RSNs. Compared with HCs, the PD patients showed decreased resting-state functional connectivity in the occipital cortex mainly and other regions including in the supplementary motor area, primary sensorimotor cortex (part of SMN and BGN), lateral occipital cortex, anterior and posterior cingulate cortex (part of DMN, CEN, and BGN) (Figure 2 and 3, p<0.05, FWE). Figures 4 shows different inter-network connectivity between PD patients and HCs. PD patients showed stronger inter-network connectivity in VN-SMN (-0.49 vs 0.01), VN-CEN (-0.39 vs 0), VN-BGN (0.55 vs 0.3) than HCs, but reduced connectivity in VN-DMN (0.49 vs 0.81). This finding is consistent with Yu et al’s work 5 and it may indicate the visual aids in the movement of PD patients. PD patients also showed higher connectivity in the SMN-CEN (0.71 vs 0.51) and SMN-BGN (-0.67 vs -0.32), but reduced connectivity in the SMN-DMN (0.13 vs 0.22) compared to the HCs. The global signal showed reduced correlations with most regions in the PD patients compared to the HCs but except the VN (-0.33 vs -0.28).Discussion
In this study, we investigated the existence of resting-state brain network abnormalities in PD patients. Our preliminary work showed the reduced functional connectivity in the visual network and its altered connection with other RSNs including DMN and SMN in PD patients compared to HCs. In addition, the correlations between global and regional networks were reduced mostly in the PD patients. The abnormal neural activity may provide new biomarkers to investigate the PD pathology and treatment. However, the repeatability of the above findings requires further investigations.Acknowledgements
This work is supported in part by the Alzheimer’s Association through AARF-18-566347, the Fundamental Research Funds for the Central Universities, MOE Frontier Science Center for Brain Science & Brain-Machine Integration, Zhejiang University, and Zhejiang Medical and health science and Technology project (2018KY190, 2021KY420).References
- Campbell MC, Koller JM, Snyder AZ, Kotzbauer PT. CSF proteins and resting-state functional connectivity in Parkinson disease. Neurology. 2015;84:2413–2421.
- Wolters AF, van de Weijer SCF, Leentjens AFG, Duits AA, Jacobs HIL, Kuijf ML. Resting-state fMRI in Parkinson’s disease patients with cognitive impairment: A meta-analysis. Park. Relat. Disord. [Internet]. 2019;62:16–27. Available from: https://doi.org/10.1016/j.parkreldis.2018.12.016
- Zhao L, Alsop DC, Detre JA, Dai W. Global fluctuations of cerebral blood flow indicate a global brain network independent of systemic factors. J. Cereb. Blood Flow Metab. 2019;39:302–312.
- Beckmann C, Mackay C, Filippini N, Smith S. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. Neuroimage. 2009;47:S148.
- Yu Q, Li Q, Fang W, Wang Y, Zhu Y, Wang J, et al. Disorganized resting-state functional connectivity between the dorsal attention network and intrinsic networks in Parkinson’s disease with freezing of gait. Eur. J. Neurosci. 2021;54:6633–6645.
Figures
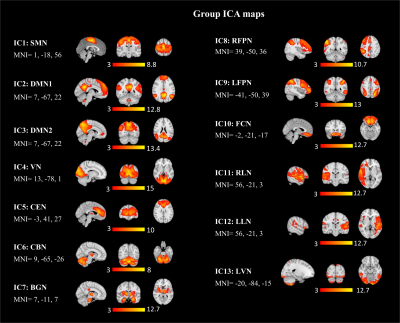
Figure 1. Thirteen group ICA
maps identified from 20 healthy controls and 37 PD patients.
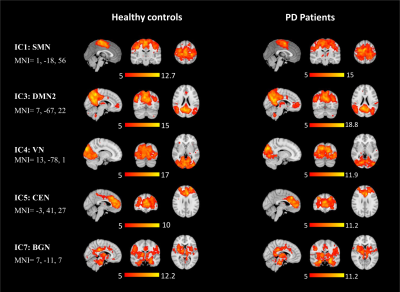
Figure 2. Spatial maps of SMN, DMN, VN, CEN, and, BGN in healthy controls and PD
patients (one-sample t-test, p<0.05, FWE).
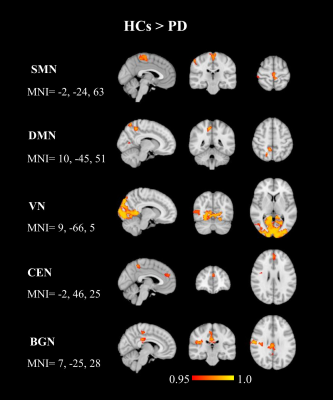
Figure 3. Regions of decreased resting-state connectivity in the SMN, DMN, VN,
CEN, and BGN in PD patients compared to HCs
(two-sample t-test, p<0.05, FWE).
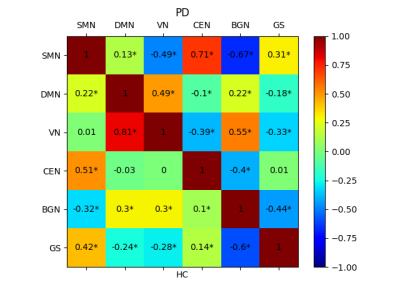
Figure 4. Inter-network correlation matrix for patients
with Parkinson’s disease (top) and healthy controls (bottom). SMN, sensorimotor
network, DMN, default mode network; VN, visual network; CEN, central executive
network; BGN, basal ganglia network; GS, global signal. * stands for p< 0.001.
DOI: https://doi.org/10.58530/2022/2958