2957
Automated volumetry of amygdala subregions in Parkinson’s disease patients1the First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
Cognitive impairment is very common in patients with Parkinson’s disease (PD). The amygdala in the limbic system of midbrain has been proved to play a unique role in cognitive function. However, there are almost no studies on changes in amygdala structure, especially the volume of amygdala subregions, in PD patients. In this study, automated volumetry of amygdala subregions in PD patients was performed and compared with healthy controls, and found that PD patients have amygdala subregions atrophy, especially in bilateral accessory basal nucleus, bilateral cortical nucleus and right medial nucleus.
Summary of Main Findings
In this study, automated volumetry of amygdala subregions in PD patients was performed and compared with healthy controls, and found that PD patients have amygdala subregions atrophy, especially in bilateral accessory basal nucleus, bilateral cortical nucleus and right medial nucleus.Introduction
PD is a common neurodegenerative disease. Cognitive impairment,as a non-motor symptom,is frequently observed in PD. The amygdala in the limbic system of the midbrain plays an important role in emotional processing, but many studies have found that the amygdala also plays a unique role in cognitive impairment. However, there are almost no studies on changes in amygdala structure, especially the volume of amygdala subregions, in PD patients. In this study, we aim to use an automatic amygdala segmentation method to determine if there was a change in whole or subfield amygdala volume in PD patients.Methods
56 clinically confirmed PD patients (mean age: 60.30 ± 13.45, range from 17 to 89 years; 22 females, 24 males) and 51 healthy control subjects (HCs) (mean age: 65.95 ± 8.77, range from 47 to 83 years; 25 females, 30 males) were recruited. All subjects were right-handed. Informed consent was acquired from each subject. Participants were scanned on a 3.0 T MR scanner (Ingenia CX, Philips Healthcare, the Netherlands) with a 32-channel receive-only head coil. Whole-brain, T1-weighted multi-shot turbo field echo (MS-TFE) was acquired: repetition time /echo time = 6.6/3.0ms, field of view = 256 × 256 mm, flip = 12°, 188 sagittal slices, and 1 mm3 spatial resolution. Automated volumetric measurement of the amygdala subregions was done using FreeSurfer 7.0 with amygdala subregions module on the T1-weighted image. The amygdala was segmented, bilaterally, into the lateral nucleus,basal nucleus,accessory basal nucleus,anterior amygdaloid area(AAA),central nucleus,medial nucleus,cortical nucleus, corticoamygdaloid transition area, and paralaminar nucleus[1,4]. Data analyses were performed using SPSS 25.0, independent-samples t test was used to compare the amygdala subregion volumes between PD group and HC group. P < 0.05 was considered statistically significant. FDR correction was applied to reduce the false-positive errors.Results
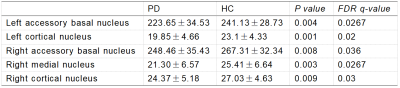
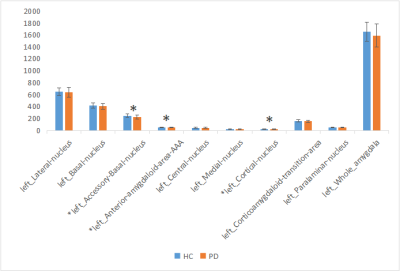
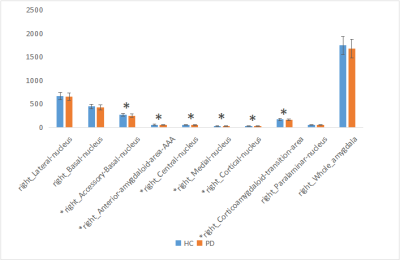
The amygdala subregions volume had a reduction trend in PD group, especially for the right side (P < 0.05) (Figure 1-2). After FDR correction, the volumes of bilateral accessory basal nucleus (P <0.01), bilateral cortical nucleus (P <0.01) and right medial nucleus (P <0.01) decreased significantly compared to HCs. The volumes of AAA, right central nucleus and right corticoamygdaloid transition area of PD group were decreased than that of HC group, but there was no significant difference after FDR correction.Discussion
This study investigated the volumes of amygdala substructures in patients with PD. We observed the reduced volumes of bilateral accessory basal nucleus, bilateral cortical nucleus and right medial nucleus in patients with PD while compared with HC group. Study showed that cognitive decline was related to amygdala connectivity[2,3]. Memory is controlled by different and interacting brain regions, while internal connections are formed between the amygdala subregions[7]. First of all, the amygdala is related to emotional significant long-term memory, and this regulatory mechanism of the amygdala may be realized through structural and functional connections with the visual cortex, hippocampus and other brain tissues. The amygdala has rich neural connections, which receive incoming information from all sensory channels, and the information from various sensory channels are integrated at different processing levels. The amygdala also has a wide range of output pathways, including the cerebral cortex, hypothalamus and many brainstem structures. The above neuroanatomical features and complex loop connections provide a fine anatomical basis for the amygdala to regulate memory[5], those circuits’ functions and interaction need further investigation. In addition, accessory basal nucleus is the main afferent connection of the central nucleus and transition zones[6]. Therefore, we speculate that amygdala subregion (mainly bilateral accessory basal nucleus,bilateral cortical nucleus and right medial nucleus) atrophy in PD patients may mainly affect emotional significant long-term memory function. However, it is not clear which subregions of amygdala affects cognitive function at present, and further research is needed to clarify it.Conclusion
Patients with PD have amygdala subregion atrophy, especially in bilateral accessory basal nucleus,bilateral cortical nucleus and right medial nucleus.Acknowledgements
No acknowledgement found.References
[1] Iglesias, J. E , Kouwe V D , et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas.
[2] Roy A K , Shehzad Z , Margulies D S , et al. Functional connectivity of the human amygdala using resting state fMRI[J]. Neuroimage, 2009, 45(2):614-626.
[3] Yao H X, Ning-Yu A N , Zhang X . Study on longitudinal alteration of amygdala functional connectivity in the amnestic mild cognitive impairment by resting-state fMRI[J]. Radiologic Practice, 2014.
[4] Pan H E , Qiang M A . Study on the Relationship between Medial Temporal Lobe Changes and Amnestic Mild Cognitive Impairment Based on VBM-DARTEL and FreeSurfer[J]. Hebei Medicine, 2019.
[5] Liu D, Yao SJ. Regulation of emotional significant long-term memory consolidation in amygdaloid nucleus [J]. Chinese Journal of Behavioral Medicine and Brain Science,2006, 015(007):664-666.
[6] Fudge J L , Tucker T . Amygdala projections to central amygdaloid nucleus subdivisions and transition zones in the primate[J]. Neuroscience, 2009, 159(2):819-841.
[7]Ying,Yang,Jian-Zhi,Wang.From Structure to Behavior in Basolateral Amygdala-Hippocampus Circuits.[J].Frontiers in neural circuits,2017,11:86.