2956
Gray and white matter alterations in different predominant side and type of motor symptom in Parkinson’ disease1Radiology, The second affiliated hospital, Zhejiang University school of medicine, Hangzhou, China, 2GE Healthcare, Shanghai, China
Synopsis
It has been overlooked in clinical that there exists a complex relationship between laterality and motor symptoms in Parkinson’s disease (PD) and these factors may influence their motor and nonmotor symptoms in together. Here we found PD with left-side akinetic/rigid (L-AR) signs performed worst in clinical manifestations. And the underlying brain structure alterations was revealed severer damage in anterior cingulate and its connected fibers of right hemisphere by a multimodal imaging approach including cortical thickness and diffusion-weighted MRI measures.
Introduction
There is an intricate relationship between laterality and motor symptoms of Parkinson’s disease (PD) and the combination of these factors may influence the motor progression and cognitive deterioration [1-4]. Nonetheless, very few studies have examined the influence of side and type of motor signs to the presence of motor and nonmotor symptoms in PD so far, let alone the related mechanism research. In this study, we aim to explore the clinical characteristics and underlying alterations of brain structure in PD with different side and type of symptoms.Methods
We recruited 322 right-handed subjects including 262 PD patients and 60 healthy controls (HCs). Patients were classified into four groups, according to their predominant side and type of motor signs: left akinetic/rigid (L-AR), left tremor (L-TD), right akinetic/rigid (R-AR), and right tremor-dominant (R-TD)[5,6]. All subjects were scanned in a 3.0 Tesla MRI machine (GE Discovery 750) equipped with an 8-channel head coil. Three dimensions T1-weighted images were acquired using a Fast-Spoiled Gradient Recalled sequence and cortical thickness and subcortical volume features were extracted through FreeSurfer (v6) pipeline[7]. DTI images were acquired using a spin echo-echo planar imaging sequence with 30 gradient directions (b value = 1,000 s/m2) and diffusion parameters (i.e., fractional anisotropy [FA], mean diffusivity [MD], axial diffusivity [AD], and radial diffusivity [RD]) were calculated by Tract-based spatial statistics (TBSS)[8,9].Results
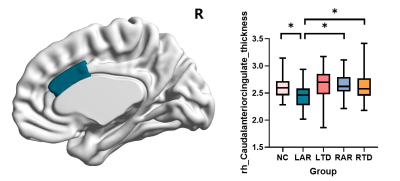
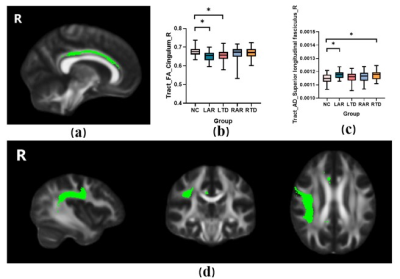
The motor symptoms measured with UPDRS-total (P=0.002), UPDRS-Ⅲ(P=0.002) and Hoehn and Yahr staging (P=0.001) were significantly higher in the LAR group among the PD groups. Compared with NCs, the PD groups showed increased in HAMD and HAMA scales (PHAMD<0.001, PHAMA=0.005). The education and MMSE was slightly lower in the patients who had PD with predominant left-sided signs (Peducation=0.043, PMMSE=0.075). The differences in cortical thickness and subcortical volume among five groups were investigated (Figure 1). It showed a significant reduction in cortical thickness of right caudal-anterior-cingulate in the LAR group. FA, MD, AD and RD values of WM tracts were compared among five groups (Figure 2). LAR group showed lowest mean FA values in right cingulum tracts and highest mean AD values of right superior longitudinal fasciculus tracts.Conclusions
Patients with LAR signs have most severe clinical symptoms and may result from severer damage in anterior cingulate and its connected fibers of right hemisphere. This indicates the importance of accurate classification of subtypes in clinical. Further studies should be performed to explore the various clinical reactivity of these subtypes.Acknowledgements
This work is supported by the 13th Five-year Plan for National Key Research and Development Program of China (Grant No.2016YFC1306600), the National Natural Science Foundation of China (Grant No. 81971577, 81571654, 81701647 and 81771820),the Fundamental Research Funds for the Central Universities of China(Grant No. 2017XZZX001-01). The authors appreciate the clinical assistance from other neurologists in the Department of Neurology, the Second Affiliated Hospital of Zhejiang University School of Medicine.References
1. Katzen HL, Levin BE, Weiner W: Side and type of motor symptom influence cognition in Parkinson's disease. Mov Disord 2006, 21(11):1947-1953.
2. Baumann CR, Held U, Valko PO, Wienecke M, Waldvogel D: Body side and predominant motor features at the onset of Parkinson's disease are linked to motor and nonmotor progression. Mov Disord 2014, 29(2):207-213.
3. Seichepine DR, Neargarder S, Davidsdottir S, Reynolds GO, Cronin-Golomb A: Side and type of initial motor symptom influences visuospatial functioning in Parkinson's disease. J Parkinsons Dis 2015, 5(1):75-83.
4. Rodriguez-Violante M, Cervantes-Arriaga A, Villar-Velarde A, Corona T: Relationship between the type and side of motor symptoms with the prevalence of non-motor symptoms in Parkinson's disease. Neurologia 2011, 26(6):319-324.
5. Lee EY, Sen S, Eslinger PJ, Wagner D, Kong L, Lewis MM, Du G, Huang X: Side of motor onset is associated with hemisphere-specific memory decline and lateralized gray matter loss in Parkinson's disease. Parkinsonism Relat Disord 2015, 21(5):465-470.
6. Kang GA, Bronstein JM, Masterman DL, Redelings M, Crum JA, Ritz B: Clinical characteristics in early Parkinson's disease in a central California population-based study. Mov Disord 2005, 20(9):1133-1142.
7. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002,33(3):341-55.
8. Guo T, Guan X, Zhou C, Gao T, Wu J, Song Z, Xuan M, Gu Q, Huang P, Pu J et al: Clinically relevant connectivity features define three subtypes of Parkinson's disease patients. Hum Brain Mapp 2020, 41(14):4077-4092.
9. Juttukonda MR, Franco G, Englot DJ, Lin YC, Petersen KJ, Trujillo P, Hedera P, Landman BA, Kang H, Donahue MJ et al: White matter differences between essential tremor and Parkinson disease. Neurology 2019, 92(1):e30-e39.
Figures