2953
Structural and functional changes of the cerebellum in patients with Parkinson's disease: a VBM and rs-fMRI study1Lanzhou University Second Hospital, Lanzhou, China, 2Philips Healthcare, Xi'an, China
Synopsis
To investigate the structural and functional alterations of cerebellum in patients with Parkinson's disease (PD) using voxel-based morphometry and resting-state functional MRI. We analyzed alterations gray matter volume (GMV), regional homogeneity (ReHo), amplitude of low-frequency fluctuation (ALFF) in the cerebellum of PD patients. Furthermore, the correlation analysis between GMV, ALFF, ReHo values and clinical scale scores was performed. Our results showed that the structure and function of the cerebellum of PD patients are significantly different from healthy controls (HC), and these changes are significantly correlated with clinical scales scores, which suggested that the cerebellum plays an important role in PD.
Introduction
Parkinson's disease (PD) is the most common neurodegenerative disorder after Alzheimer’s disease and expected to impose an increasing social and economic burden on societies1. It’s recognized that pathological mechanism is the selective loss of dopaminergic neurons in the substantia nigra pars compacta, leading to the dysfunction of striatum-thalamus-cortex (STC) circuit2, while the cerebellum is often ignored. Pathophysiological and atrophic changes in the cerebellum are documented in PD3. Up to now, on the one hand, the specific role of the cerebellum in PD is still unclear. On the other hand, there are few studies on the changes in the structure and function of the cerebellum in PD patients, and the existing studies show great heterogeneity. The purpose of this study was to explore the alterations of cerebellar structure and function in patients with PD by using voxel-based morphometry (VBM) and resting-state functional MRI (rs-fMRI) method, and analyze the clinical significance of these alterations. The proposed method may provide complementary information to find cerebellar imaging biomarkers of PD .Methods
Forty-six PD patients (24 males, 22 females; 66.6±10.2 yrs; level of education 10.10±3.98 yrs) were recruited from the Second Hospital of Lanzhou University. All patients satisfied the United Kingdom Parkinson’s Disease Society Brain Bank criteria4. A total of forty-six age-, sex-, and educational -matched HC were recruited. PD patients were assessed on the motor section of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS part III). All participants underwent Montreal Cognitive Assessment (MoCA) as measures of general cognition. Imaging was conducted on a 3T MR scanner (Ingenia CX, Philips Healthcare, the Netherlands). Whole brain high resolution 3D T1-weighted sequences were acquired as follows: sagittal orientation, 192 slices, 256x256 in-plane matrix, TR/TE = 2000/2.7ms, 1x1x1 mm3 spatial resolution. T2*-weighted echo planar functional images were acquired with 36 axial slices covering the whole brain, 128x128 in-plane matrix, TR/TE= 2000/30ms, and 3x3x3 mm3 spatial resolution. The structural and functional images were preprocessed by using SPM12 (https://www.fil.ion.ucl.ac.uk/spm/) and DPABI (http://www.rfmri.org/dpabi) software. Gray matter volume (GMV), regional homogeneity (ReHo) and amplitude of low-frequency fluctuation (ALFF) of all subjects were obtained. Then two-sample t test was performed to find the group difference. Finally, Spearman’s correlation analysis was calculated between the UPDRS-III/MoCA scores and GMV, ALFF, ReHo.Results
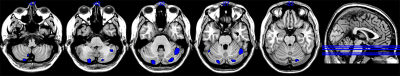
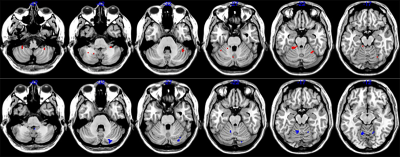
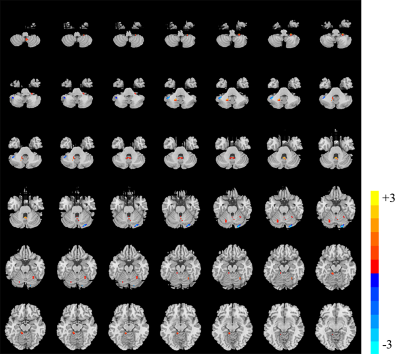
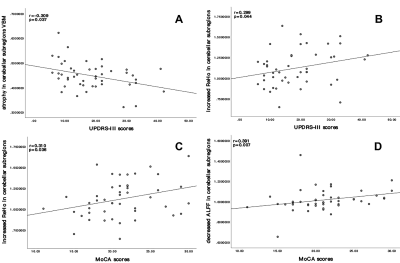
Compared with the HC, atrophy in cerebellar subregions was apparent in the PD patients which included the cluster cerebellar subregions bilateral cerebellum_Crus1 and cerebellum_Crus2 (Fig. 1). There were both decreased and increased subregions of ALFF in PD patients in comparison to HC. (Fig 2). The PD patients had increased ReHo in the bilateral cerebellum_6, cerebellum_3_L, cerebellum_4_5_L, cerebellum_9_R, Vermis_9, and decreased ReHo in the cerebellum_Crus1_R and cerebellum_Crus2_L (Fig 3). The Spearman analysis showed that there was a negative correlation between UPDRS-III scores and decreased GMV (r = -0.309, p = 0.037), and a positive correlation with increased ReHo (r = 0.299, p = 0.044) in the cerebellum in patients with PD (Fig. 4). In addition, a positive correlation between the MoCA scores and the increased ReHo (r = 0.310, p = 0.036) and decreased ALFF (r = 0.391, p =0.007) in the cerebellum in PD patients (Fig. 4).Discussion
Our results showed that there were structural and functional changes in the cerebellum of PD patients, and there was overlap between the subregions of structural changes and functional changes. The findings described here revealed a combination of cerebellar structure and function abnormalities in PD. We observed an inverse correlation between the UPDRS III score and the extent of cerebellar atrophy, but it is positively correlated with increased ReHo. The cerebellum's role in motor function is well recognized5, we speculate that the increase of ReHo in the cerebellar subregion is a compensatory activation in order to maintain relatively normal motor function, while the atrophy of the cerebellar subregion is pathological. In addition, MoCA scores were positively correlated with increased ReHo and decreased ALFF, this alterations to the spontaneous neural activity of the cerebellum may also play a compensatory change in patients with PD, in order to maintain relatively normal cognitive function. Further investigations are needed to clarify the mechanism of overlapped subregions of structural and functional changes in cerebellum and its effect to PD related neuropathological alterations.Conclusion
Anatomical, pathophysiological and clinical evidences suggested that the cerebellum may contribute substantially to the clinical symptoms of PD. Our study indicated that the major role of the cerebellum in PD includes two aspects, pathological and compensatory effects. The compensatory effect may help patients to maintain relatively normal motor and cognitive function. The above research is expected to find the imaging biomarkers of the cerebellum for the early diagnosis of PD.Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 81960309).References
[1]DE LAU L M, BRETELER M M. Epidemiology of Parkinson's disease [J]. Lancet Neurol, 2006, 5(6): 525-35.
[2]FOFFANI G, OBESO J A. A Cortical Pathogenic Theory of Parkinson's Disease [J]. Neuron, 2018, 99(6): 1116-28.
[3]WU T, HALLETT M. The cerebellum in Parkinson's disease [J]. Brain, 2013, 136(Pt 3): 696-709.
[4]MARTINEZ-MARTIN P, FALUP-PECURARIU C, RODRIGUEZ-BLAZQUEZ C, et al. Dementia associated with Parkinson's disease: applying the Movement Disorder Society Task Force criteria [J]. Parkinsonism Relat Disord, 2011, 17(8): 621-4.
[5]KOZIOL L F, BUDDING D, ANDREASEN N, et al. Consensus paper: the cerebellum's role in movement and cognition [J]. Cerebellum, 2014, 13(1): 151-77.
Figures