2952
Region-specific neurovascular decoupling associated with cognitive decline in Parkinson’s disease1Nanjing First Hospital, Nanjing Medical University, Nanjing, China, 2GE Healthcare, MR Research China, Beijing, China, 3Clinical Medical College, Yangzhou University, Yangzhou, China
Synopsis
Cognitive deficits are prominent non-motor symptoms in Parkinson’s disease (PD) and have been involved with neurovascular unit. However, sufficient neuroimaging studies are lacked investigating the associated modulating mechanisms. This study aimed to identify the contribution of neurovascular decoupling to the pathogenesis of cognitive decline in PD by integrating informative data obtained from the blood oxygen level-dependent and arterial-spin-labeling approaches. The involvement of neurovascular decoupling in cognitive impairment in PD is regionally specific and most prominent in the visual-spatial cortices, which could potentially provide a complementary understanding for the pathophysiological mechanisms underlying cognitive deficits in PD.
Introduction
Parkinson’s disease (PD) is an age-related neurodegenerative disorder accounting for clinical manifestations with a spectrum of nonmotor dysfunction1, 2. Notably, mild cognitive impairment in PD (PD-MCI) has been defined as a transitional stage between normal aging and dementia3. A comprehensive understanding of the pathophysiological mechanisms in PD-MCI is still needed. As the primary components of neurovascular unit (NVU), neurons and the vasculature could also be vulnerable to the involvement of neuroinflammation and oxidative stress in PD4. However, there is still a paucity of neuroimaging investigations regarding neurovascular decoupling and the consequent cognitive deficits in PD-MCI. Hence, we aimed to investigate neurovascular decoupling in PD-MCI by integrating regional homogeneity (ReHo) and cerebral blood flow (CBF) information.Materials and Methods
SubjectsA final sample consisted of 47 (26 male and 21 female) patients with normal cognition (PD-NC), 45 (24 male and 21 female) patients with PD-MCI, and 46 (24 male and 22 female) matched healthy controls (HCs) were recruited. The Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) and Hoehn-Yahr (H-Y) scale were scored for disease severity and stage of PD. The impairments in global and cognitive domains were assessed using a neuropsychological battery.
MRI experiment
MRI experiments were performed using a 3.0-tesla MRI scanner (Discovery MR750, GE, USA) with an 8-channel phased array head coil. Resting-state functional MRI (fs-MRI) of the whole brain were acquired as follows: TR, 2,000 ms; TE, 30 ms; FA, 90°; slice thickness, 4 mm without gap; FOV, 240 × 240 mm2; matrix size, 64 × 64; voxel size, 4.0 × 4.0 × 4.0 mm3; 240 time points; and total scan time, 480 s. 3D pseudo-continuous arterial-spin-labeling (ASL) sequence with the following parameters was applied: TR 10.5 ms, TE 4.9 ms, FA 111°, slice thickness 4 mm without gap, FOV 240 × 240 mm2, matrix size 128 × 128, post-label-delay 2025 ms, NEX 3, 36 slices. Total scan time was 284 s. Data analysis Functional and perfusion data were preprocessed in SPM 12 embedded in the MATLAB 2018a platform. The neurovascular coupling was quantitatively assessed by calculating the correlation coefficient between the z-ReHo map and z-CBF map across voxels at the whole-brain grey matter (GM) level. Regional neurovascular coupling was further analyzed by dividing the whole-brain GM into 90 independent functional sub-regions based on the automated anatomical labeling atlas. In addition, CBF/ReHo ratios across voxels within the GM mask were calculated with the original values of the metrics.
Statistical analysis
The UPDRS-III scores, H-Y stage and disease duration were compared using Mann-Whitney U tests. One-way analysis of variance (ANOVA) (for scores of five cognitive domains, metrics of whole-brain GM and regional neurovascular coupling) were utilized for comparisons among different groups. SPSS 22.0 software was used for the above analyses, and the significance level was set as a P value < 0.05. A voxel-wise one-way analysis of covariance within the GM mask was applied in SPM12 software for investigating differences among different groups. The significance level was set at an uncorrected voxel-wise threshold of P < 0.001 and a familywise error (FWE)-corrected cluster-wise threshold of P < 0.05. Pearson or Spearman correlation tests were further applied to explore potential associations between clusters values and clinical assessments.
Results
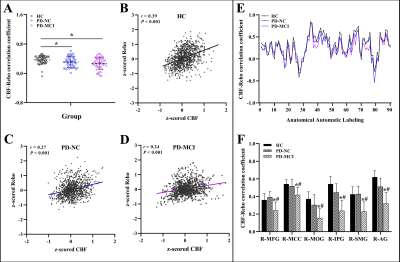
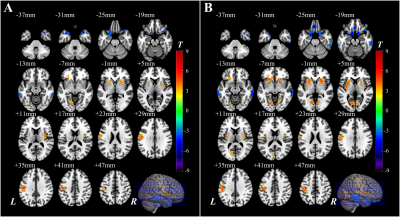
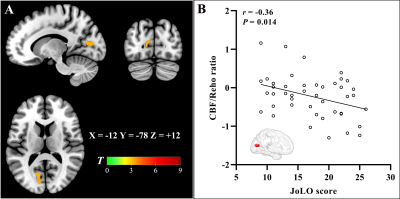
The PD-MCI patients suffered from significant impairments within the global cognition and five domains compared with other two groups (P < 0.05). Neurovascular coupling was impaired in PD-MCI patients with a decreased global CBF-ReHo correlation coefficient relative to HC subjects (P < 0.05) (Fig.1). Regional dysregulation was specific to PD-MCI group and localized to the right middle frontal gyrus, right middle cingulate cortex, right middle occipital gyrus, right inferior parietal gyrus, right supramarginal gyrus, and right angular gyrus (P < 0.05) (Fig.1). Compared with HC subjects, PD-MCI patients showed higher CBF/ReHo ratios in the bilateral lingual gyri (LG), bilateral putamen and left postcentral gyrus and lower CBF/ReHo ratios in the right superior temporal gyrus, bilateral middle temporal gyri, bilateral parahippocampal gyri and right inferior frontal gyrus (Fig.2). Relative to HC and PD-NC groups, PD-MCI group showed an increased CBF/ReHo ratio in the left LG, which was correlated with poor visual-spatial performance (r = -0.36, P = 0.014) (Fig.3).Discussion and conclusion
The current study investigated neurovascular decoupling and resultant deterioration in PD-MCI patients by integrating informative data obtained from the blood oxygen level-dependent and ASL approaches. The PD-MCI group exhibited dysregulation of NVU at the whole-brain GM level as well as regional disturbances in cognition-related cortices. In comparison with HC subjects, PD-MCI patients had increased CBF/ReHo ratios in motor-related regions and decreased ratios in the limbic system and prefrontal and temporal cortices. Specifically, a distinct neurovascular decoupling in the LG that involved visuospatial dysfunction was characterized in the PD-MCI group relative to HC and PD-NC groups. In conclusion, these imaging signatures linked to neurovascular coupling could potentially provide complementary insights into the underlying mechanisms of cognitive impairment during the progression of PD. This work illuminated the involvement of neurovascular decoupling in the neurodegenerative process in PD-MCI and emphasized the potential role of coupling metrics for investigating the mechanism of cognitive decline associated with PD.Acknowledgements
No acknowledgement found.References
1. Kalia LV, Lang AE. Parkinson's disease. Lancet 2015;386:896-912.
2. Dickson DW. Neuropathology of Parkinson disease. Parkinsonism Relat Disord 2018;46 Suppl 1:S30-S33.
3. Baiano C, Barone P, Trojano L et al. Prevalence and clinical aspects of mild cognitive impairment in Parkinson's disease: A meta-analysis. Mov Disord 2020;35:45-54.
4. Campolo M, Paterniti I, Siracusa R et al. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson's diseases in vivo model. Brain Behav Immun 2019;76:236-247.
Figures