2950
Subcortical Gray Matter Nucleus Lateralization of Amide Proton Transfer Weighted Signals in Young Healthy Subjects1Radiology, the First Affiliated Hospital of Dalian Medical University, Dalian, China, 2the First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
Amide proton transfer weighted (APTw) imaging is a novel molecular imaging technique to acquire the proton exchanged signals from the amide in proteins or peptides to water. Previous studies discovered lateralization in the brain structure and function, including the subcortical gray matter nucleus. However, it is not clear whether APTw also has lateral advantage. In this study, we applied the automatic brain segmentation method to quantify the APTw signal values of subcortical gray matter nucleus in young healthy subjects. The increased APTw signals were present in the right side of the subcortical gray matter nucleus,which may suppose related with right-handedness.
Introduction
Amide proton transfer weighted (APTw) MR imaging is a novel non-invasive molecular imaging technique. The signals in APTw are based on the amide protons content in endogenous proteins or peptides. Previous studies have shown that APTw technique is capable of diagnosis in ischemic stroke, tumors and neurodegenerative diseases [1][2][3]. Although one study on the APTw signal values of different anatomical regions in young and healthy normal brain parenchyma had been published[4], the manual ROI delineation method in this study may led to certain measurement errors. Furthermore,it is not clear whether APTw also has lateral advantage. And we believe that before using this new technology for disease research and analysis of pathophysiological mechanisms, the most important is to understand the normal APTw signal values and the side difference in advance. In this study, we aim to use an automatic segmentation method of brain tissue to quantify the APTw signal intensity in the subcortical gray matter nucleus in healthy young volunteers.Methods
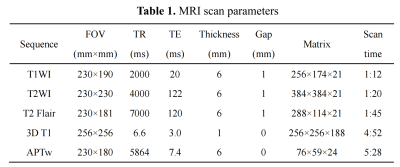
Nineteen healthy volunteers (mean age: 25.37 ± 1.49, range from 22 to 29 years; 10 females, 9 males) were recruited and all were right-handed. Informed consent was acquired from each subject. All volunteers were performed MR examination on a 3.0 T MR scanner (Ingenia CX, Philips Healthcare, the Netherlands) with a 32-channel receive-only head coil. MR protocols were performed in this study including axial T1 sequence, axial T2 sequence, axial T2 FLAIR, sagittal 3D T1 TFE and 3D TSE APTw sequence. Scan parameters were shown in Table 1.After the scanning, the unsaturated S0 image and APTw images were automatically reconstructed. Both the APTw and 3D T1-weighted data were visually inspected by two radiologists for apparent artifacts arising from subject motion and instrument malfunction. First, use the dcm2niigui software to convert the original DICOM image into 3D NIFTI format. Then, T1-weighted images of each subject were co-registered to the subject's S0 image using the SPM8 package, resulting in a co-registered T1 image (rT1) in APTw space. Utilizing SPM8 package, each subject's rT1 image was first normalized to the T1 template in Montreal Neurological Institute (MNI) space. Further, each normalized APTw image was spatially smoothed by a 6-mm full-width at half maximum Gaussian kernel to reduce the effect of misregistration in spatial normalization. Finally, use the template in the MNI space to extract the APTw signals of each region of the cerebral hemisphere. All statistical analyses were performed using the SPSS 22.0 software package. The paired-sample t test was used to analyze the APTw signals of bilateral hemispheres in the subcortical gray matter nucleus.
Results
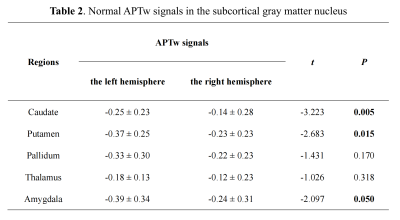
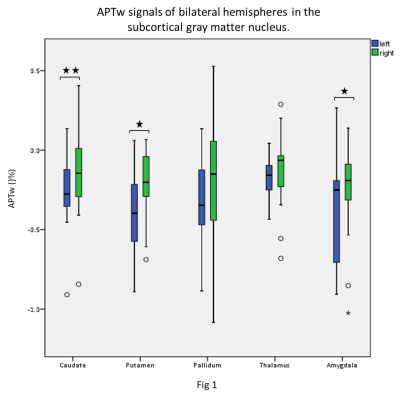
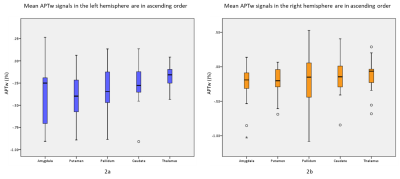
In our data, there were no apparent motion artifacts in all subjects. The averaged APTw signal intensity and standard deviation signals in different brain regions of the subcortical gray matter nucleus are presented in Table 2 and Figure 1-2. Significant elevated APTw signals in the subcortical gray matter nucleus in the right side were found than those in the left side, especially in the caudate, putamen and amygdala (P < 0.05) (Fig 1). In addition, the results showed that APTw signals in the subcortical gray matter nucleus were not impacted by the brain regions (P > 0.05) (Fig 2). Furthermore, differences between APTw signals for the left and right side were highest in the caudate (difference: 0.11%) and tend toward zero for thalamus (difference: 0.06%).Discussion
In this study, the averaged APTw signal values in different brain structures in the deep grey matter nuclei were measured in healthy young subjects. And we found that although the normal APTw signal values of subcortical grey matter nuclei structures only has statistical difference between the left and right sides in part structures, overall, the right side is higher than the left side. At present, APTw imaging research for neurodegenerative diseases is gradually increasing. Deep grey matter nuclei are involved in processing all physiological behaviors and are affected by the most prevalent neurodegenerative diseases, including Alzheimer's disease, and Parkinson's disease[3]. Our results of this study are different from previous’s. The study of Sartoretti T[4] showed that the APTw signals in the left gray matter nuclei is higher than the right side. However, they used manual ROI delineation method for measurement, while our study applied automatic segmentation method and we believe the results were more accurate. And We suspect that this difference may be related to right-handedness. As we all know, even in the same hemisphere on the left and right sides, there are different numbers of neuron structures and myelin sheath thickness, so there are certain side differences between the left and right hemispheres. Therefore, its APTw signal values may also have side advantages. However, in this study, there was no difference in APTw signal values among the gray matter nuclei on the same side. The possible reason is that the content of mobile proteins and peptides in the gray matter is similar.Conclusion
The baseline of APTw signal values in different anatomical localizations of l subcortical grey matter nuclei were measured in young healthy subjects. APTw signal values differed between left and right brain hemisphere, which may related to right brain dominance.Acknowledgements
No acknowledgement found.References
[1] Yu H, Lou H, Zou T, Wang X, Jiang S, Huang Z, Du Y, Jiang C, Ma L, Zhu J, He W, Rui Q, Zhou J, Wen Z. Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol. 2017 Nov;27(11):4516-4524. doi: 10.1007/s00330-017-4867-z.
[2] Msayib Y, Harston GWJ, Tee YK, Sheerin F, Blockley NP, Okell TW, Jezzard P, Kennedy J, Chappell MA. Quantitative CEST imaging of amide proton transfer in acute ischaemic stroke. Neuroimage Clin. 2019;23:101833. doi: 10.1016/j.nicl.2019.101833. Epub 2019 Apr 23. PMID: 31063943; PMCID: PMC6503165.
[3] Li S, Chan P, Li C, et al. Changes of Amide Proton Transfer Imaging in Multiple System Atrophy Parkinsonism Type. Front Aging Neurosci. 2020;12:572421. Published 2020 Sep 30. doi:10.3389/fnagi.2020.572421
[4] Sartoretti T, Sartoretti E, Wyss M, et al. Amide Proton Transfer Contrast Distribution in Different Brain Regions in Young Healthy Subjects. Front Neurosci. 2019;13:520. Published 2019 May 22. doi:10.3389/fnins.2019.00520
Figures