2944
Correlation between GABA+ levels in mPFC and topological characters of EEG functional connectivity network during light sleep in young adults1China Medical University, Shenyang, China, 2Department of Radiology, Shengjing Hospital of China Medical University, Shenyang, China, 3Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, USA F. M. Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4MR Scientific Marketing, Siemens Healthineers, Beijing, China
Synopsis
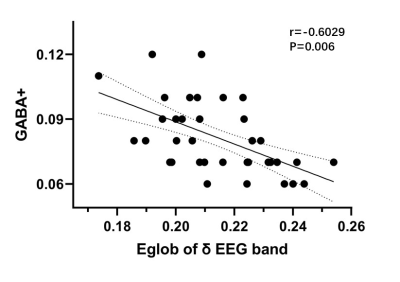
The aim of our study is to explore the relationship between GABA levels of medial prefrontal cortex (mPFC)and the topological characters of cerebral EEG functional connectivity network in young adults during sleep using Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) method. Synchronous EEG-MRS data were acquired from 37 young adults. GABA+ level in mPFC and the global topological characters of the EEG functional connectivity network were calculated. The results demonstrated a significant negative correlation between global efficiency of EEG functional connectivity network in delta bandand and GABA+ levels in mPFC during N2 sleep stage.
Introduction
GABA is the major inhibitory neurotransmitter in the human brain. It plays a crucial role in sleep initiation and maintenance [1]. Previous studies suggest that GABA levels were negatively correlated with some resting-state networks under fMRI-MRS technology during rest state[2-4]. However, the effect of GABA levels on the EEG functional connectivity during light sleep have not been investigated. The aim of our study is to explore the relationship between GABA levels of medial prefrontal cortex (mPFC)and the topological characters of cerebral EEG functional connectivity network in young adults during sleep using Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) method.Method
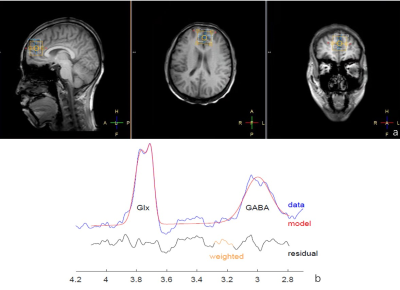
Synchronous EEG-MRS data were acquired from 37 young adults (right-handed, male: female ratio, 20:17; age, mean=24.89±1.29 years, same level of education, mean=15.03±0.69 years) using a 3T MR scanner and a 32-channel EEG system.Visual scoring of EEG data permitted sleep staging. A T1-weighted 3D TFE scan was performed first for localization of the HERMES MRS volume of interest (VOI) and subsequent tissue segmentation. HERMES MRS VOI (30 × 30 × 30 mm3) was placed in the mPFC. MRS data were postprocessed by gannet 3.0 software package,GABA+ level (GABA+ macromolecules/homocarnosine) was quantified using creatine(Cr) as concentration references.
The EEG functional connectivity network was constructed by calculating the cross-correlation between different brain regions using EEGlab software package for each participant in different EEG band respectively. Graph-theoretical network analysis was then performed to assess the global topological characters of the EEG functional connectivity network (global efficiency, local efficiency, and small-world index) by GRETNA software package. A Spearman's correlation analysis was used to explore the relationship between GABA+ levels in mPFC and the global topological characters of the EEG functional connectivity network.
Results
A significant negative correlation between global efficiency of EEG functional connectivity network in delta bandand and GABA+ levels in mPFC during N2 sleep stage (r=-0.6029, P=0.006).Discussion
This study investigated the brain functional connectivity based on EEG data collected during light sleep stage of healthy young adults and GABA+ levels based on MRS data. Our study enabled us to show that the mPFC GABA+ levels were negatively correlated with the functional connection in light sleep state. The medial prefrontal lobe is the main component of the default mode network (DMN)[5]. Studies showed DMN has been frequently associated with insomnia among the resting-state functional networks because over-activation of the DMN could be related to hyperarousal in insomnia. Therefore, the GABA+ levels in the medial prefrontal lobe detected in the current study provide supporting evidence on the mechanism of over-activation in the DMN. This was consistent with previous waking studies in healthy adults showing associations between GABA+ levels in the modulation of resting-state functional connectivity. The findings indicated repetitive transcranial magnetic stimulation(rTMS) could change the GABA+ levels to treat subjects with sleep disorders, our study provided more intuitive evidence for this mechanism.Conclusion
Our study revealed the correlation between global efficiency of EEG functional connectivity network in delta bandand and GABA+ levels in mPFC during N2 sleep stage.Acknowledgements
This study applies tools developed under NIH R01 EB016089 and P41 EB015909; RAEE also receives salary support from these grants.References
1. Park, S., et al., Shorter sleep duration is associated with lower GABA levels in the anterior cingulate cortex. Sleep Med, 2020. 71: p. 1-7.
2. Verweij, I.M., et al., Sleep deprivation leads to a loss of functional connectivity in frontal brain regions. BMC Neurosci, 2014. 15: p. 88.
3. Levar, N., et al., Anterior cingulate GABA and glutamate concentrations are associated with resting-state network connectivity. Sci Rep, 2019. 9(1): p. 2116.
4. Chen, X., et al., Regional GABA Concentrations Modulate Inter-network Resting-state Functional Connectivity. Cereb Cortex, 2019. 29(4): p. 1607-1618.
5. Raichle, M.E., The brain's default mode network. Annu Rev Neurosci, 2015. 38: p. 433-47.
Figures