2942
Alterations in ALPS index and choroid plexus volume after lumboperitoneal shunt in patients with idiopathic normal pressure hydrocephalus1Department of Radiology, Juntendo University Graduate School of Medicine, Bunkyo-ku, Japan, 2Department of Innovative Biomedical Visualization, Graduate School of Medicine, Nagoya University, Nagoya, Japan, 3Department of Neurosurgery, Juntendo University Faculty of Medicine, Bunkyo-ku, Japan, 4Department of Neurosurgery, Juntendo Tokyo Koto Geriatric Medical Center, Koto-ku, Japan, 5Department of Radiology, Nagoya University Graduate School of Medicine, Nagoya, Japan
Synopsis
Idiopathic normal pressure hydrocephalus (iNPH) is a condition resulting from impaired cerebrospinal fluid (CSF) absorption and excretion that is characterized by a triad of symptoms comprising cognitive decline, gait disturbance, and urinary incontinence. By improving CSF turnover through shunt surgery, symptoms of iNPH can become less severe. However, many mysterious points still exist in the mechanism of CSF dynamics in patients with iNPH. We examined alterations in ALPS index and choroid plexus volume after lumboperitoneal shunt (LPS) surgeries in patients with iNPH. Our results showed improvements in ALPS index and choroid plexus volume in these patients after LPS.
Introduction
Idiopathic normal pressure hydrocephalus (iNPH) is associated with gait dysfunction, cognitive impairment, and urinary incontinence. Lumboperitoneal shunt (LPS) can improve these symptoms¹. Patients with iNPH have impaired cerebrospinal fluid (CSF) absorption and excretion, thereby accumulating amyloid-β in the brain². The accumulation of amyloid-β is a characteristic feature of both iNPH and Alzheimer’s disease, which is a pathological finding that correlates with poor shunt responsiveness in iNPH patients³. The accumulation of interstitial amyloid-β also relates to the clearance dysfunction in the brain parenchyma⁴. The analysis along the perivascular space (ALPS) index is a noninvasive method adopted to measure the water diffusivity along the perivascular space in vivo⁵. The ALPS index partly evaluates the function of the CSF/interstitial fluid exchange pathway. Besides, choroid plexus (CP) has been hypothesized to secrete CSF, and its compositions are largely controlled through a blood-CSF barrier in the CP⁶. However, many mysterious points still exist in the mechanism of CSF dynamics in patients with iNPH. Therefore, we evaluated alterations in the ALPS index and CP volume between pre- and post LPS in patients diagnosed with iNPH.Methods
Study participantsWe included nine subjects diagnosed with iNPH (Table 1).
MR imaging acquisition
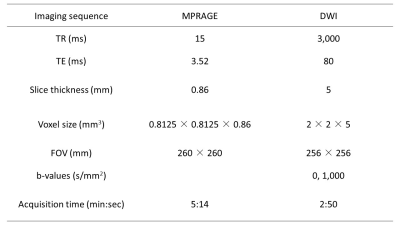
All participants were subjected to 3 T MRI scanner (Achieva Quasar Dual; Philips Medical Systems, Best, The Netherlands) before and after LPS placement. Structural T1-weighted images were also acquired using a sagittal 3D MPRAGE sequence. DWI with 32 non-collinear directions was acquired using a single-shot spin-echo echo-planar imaging sequence. Acquisition parameters of MPRAGE and DWI are presented in Table 2.
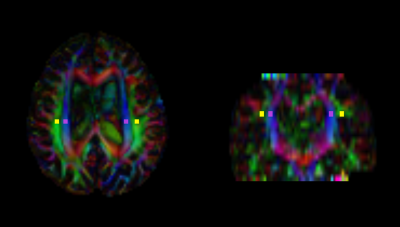
ALPS index calculation
DWI data were processed using the FMRIB Software Library (FSL) version 6.0⁷. DWI data were corrected for susceptibility-induced geometric distortions, eddy current distortions, and inter-volume subject motion using EDDY and TOPUP toolboxes⁸. Diffusivity maps of each subject in the x-axis (right-left; Dxx), y-axis (anterior-posterior; Dyy), and z-axis (inferior-superior; Dzz) were subsequently obtained. Fractional anisotropy (FA) maps of all participants were created and aligned as well, using the FSL’s linear and nonlinear registration tools. By using each subject’s color-coded FA map, we manually placed 5-mm-diameter square ROIs in projection and association areas at the level of lateral ventricle body (Figure 1). The resulting ROIs registered to the same FA template. X-, y-, and z-axes diffusivity values within ROIs were obtained for each participant, after which the ALPS index was calculated as a ratio of the mean x-axis diffusivity in the projection area (Dxxproj) and x-axis diffusivity in the association area (Dxxassoc) to the mean y-axis diffusivity in the projection area (Dyyproj) and z-axis diffusivity in the association area (Dzzassoc) as follows⁵: ALPS index = (Dxxproj ∔ Dxxassoc) / (Dyyproj ∔ Dzzassoc).
We calculated the mean ALPS index of the left and right hemispheres.
Choroid plexus volume calculation
We automatically estimated structural volumes using the FreeSurfer 6.0. Subsequently, images were manually checked for quality and inspected for motion correction, then the CP volumes of the left and right lateral ventricles were summed.
Statistical analysis
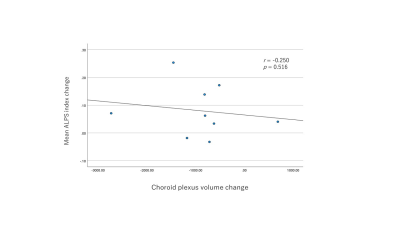
Statistical analysis was conducted using SPSS 27.0 (IBM Corporation). Mean ALPS indices and CP volumes in the pre- and post-operative iNPH groups were compared using the Wilcoxon signed-rank test. A p-value < 0.05 was considered statistically significant. Additionally, we calculated changes in the mean ALPS index and CP volume between pre- and post LPS. Then, the association between these changes in the mean ALPS index and CP volume was evaluated using Spearman’s correlation coefficients.
Results
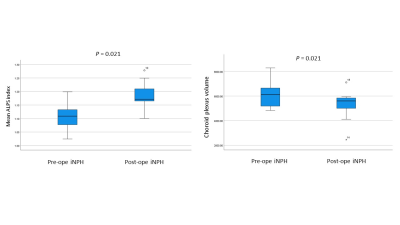
The mean ALPS index of post-operative iNPH subjects was significantly higher than that of pre-operative iNPH subjects (p = 0.021) (Figure 2a). Alternatively, the CP volume of post-operative iNPH subjects was significantly lower than that of pre-operative iNPH subjects (p = 0.021) (Figure 2b). Results also showed that the mean ALPS index changes between pre- and post LPS were not significantly correlated with that of the CP volume changes (Figure 3).Discussion
Our study is the first report to examine alterations in the ALPS index and CP volume of iNPH patients after LPS. Two previous findings used ALPS methods to evaluate the clearance system of iNPH. Yokota et al⁹ showed that ALPS indices were lower in both the pseudo-iNPH and iNPH patients than in healthy controls. Bae et al¹⁰ investigated whether the ALPS index was significantly lower in patients with iNPH than in controls (p < 0.0001). As observed, the ALPS index was also significantly lower in the iNPH group who did not show treatment response through diagnostic CSF drainage (lumbar puncture of 50cc) compared to those who showed symptomatic improvements (p < 0.0001). Besides, a previous study reported that the CP and ventricles showed a decline in all aspects of their functioning with aging¹¹, such as a drop in CSF secretion and protein synthesis, worse CSF drainage, and increased ventricular volume¹². The CP functional decline have also been indicated to be even more pronounced in pathologies, such as iNPH and Alzheimer’s disease¹³. Some previous reports exist, which showed CP enlargements during chronic pain, stroke¹³, and schizophrenia. Our study showed CP volume improvements after LPS in patients with iNPH.Conclusion
Our results showed improvements of ALPS index and CP volume in patients previously diagnosed with iNPH after LPS.Acknowledgements
This
work was supported by a Research Grant from the Ministry of Health, Labor and
Welfare of Japan (2014-Nanchi-General-052), MEXT-Supported Program for the
Private University Research Branding Project, and the JSPS KAKENHI (Grant
Number: 20K16737).
References
1. Nakajima M, Miyajima M, Ogino I, et al. Use of External Lumbar Cerebrospinal Fluid Drainage and Lumboperitoneal Shunts with Strata NSC Valves in Idiopathic Normal Pressure Hydrocephalus: A Single-Center Experience. World Neurosurgery. 2015;83(3):387-393.
2. Reeves B, Karimy J, Kundishora A, et al. Glymphatic System Impairment in Alzheimer’s Disease and Idiopathic Normal Pressure Hydrocephalus. Trends in Molecular Medicine. 2020;26.
3. Abu Hamdeh S, Virhammar J, Sehlin D, Alafuzoff I, Cesarini KG, Marklund N. Brain tissue Aβ42 levels are linked to shunt response in idiopathic normal pressure hydrocephalus. J Neurosurg. 2018;130(1):121-129.
4. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111.
5. Taoka T, Masutani Y, Kawai H, et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn J Radiol. 2017;35(4):172-178.
6. Johansson PA, Dziegielewska KM, Liddelow SA, Saunders NR. The blood-CSF barrier explained: when development is not immaturity. Bioessays. 2008;30(3):237-248.
7. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782-790.
8. Yamada H, Abe O, Shizukuishi T, et al. Efficacy of Distortion Correction on Diffusion Imaging: Comparison of FSL Eddy and Eddy_Correct Using 30 and 60 Directions Diffusion Encoding. PLoS ONE. 2014;9(11):e112411.
9. Yokota H, Vijayasarathi A, Cekic M, et al. Diagnostic Performance of Glymphatic System Evaluation Using Diffusion Tensor Imaging in Idiopathic Normal Pressure Hydrocephalus and Mimickers. Curr Gerontol Geriatr Res. 2019;2019:5675014.
10. Bae YJ, Choi BS, Kim JM, Choi JH, Cho SJ, Kim JH. Altered glymphatic system in idiopathic normal pressure hydrocephalus. Parkinsonism Relat Disord. 2021;82:56-60.
11. Vandenbroucke RE. A Hidden Epithelial Barrier in the Brain with a Central Role in Regulating Brain Homeostasis. Implications for Aging. Ann Am Thorac Soc. 2016;13 Suppl 5:S407-s410.
12. Redzic ZB, Preston JE, Duncan JA, Chodobski A, Szmydynger-Chodobska J. The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol. 2005;71:1-52.
13. Egorova N, Gottlieb E, Khlif MS, Spratt NJ, Brodtmann A. Choroid plexus volume after stroke. Int J Stroke. 2019;14(9):923-930.
Figures
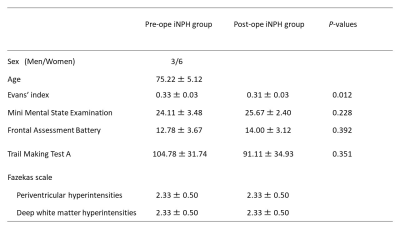
Table 1. Demographic characteristics of the participants.
Data are presented as mean ± standard deviation.